当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
pH of Single and Double Salt SO3H-Functionalized Brønsted Acidic Ionic Liquids
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-04-21 , DOI: 10.1021/acs.jced.0c01026 Zhenxing Song 1, 2 , Xianbao Cui 1, 2 , Tianyang Feng 1 , Ying Zhang 1 , Xuemei Zhang 1 , Jie He 1 , Jixiao Wang 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-04-21 , DOI: 10.1021/acs.jced.0c01026 Zhenxing Song 1, 2 , Xianbao Cui 1, 2 , Tianyang Feng 1 , Ying Zhang 1 , Xuemei Zhang 1 , Jie He 1 , Jixiao Wang 1, 2
Affiliation
|
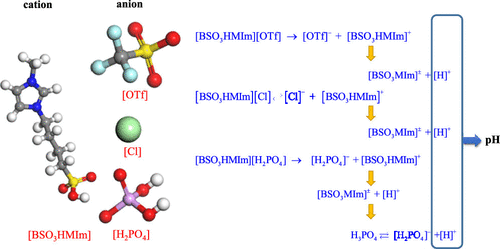
Acidic ionic liquids are widely utilized as catalysts in acid-catalyzed reactions. The acidity of SO3H-functionalized Brønsted acidic ionic liquids (S-BAILs) in aqueous solutions was systematically investigated by their pH values. The pH values of single and double salt S-BAILs in aqueous solutions were measured by a pH meter. The dissociation mechanisms of single and double salt S-BAILs in aqueous solutions were proposed. The methods to predict the pH values of single and double salt S-BAILs were also proposed based on the dissociation mechanisms, and the calculated pH values agreed well with the experimental results. The dissociation mechanism of S-BAILs in aqueous solutions is further explained by the dissociation fraction of ionic liquids, and a method to estimate the dissociation fraction of S-BAILs in dilute aqueous solutions was proposed.
中文翻译:

单盐和双盐SO 3 H-官能化布朗斯台德酸性离子液体的pH值
酸性离子液体被广泛用作酸催化反应中的催化剂。SO 3的酸度H官能化的布朗斯台德酸性离子液体(S-BAILs)在水溶液中的pH值得到了系统的研究。用pH计测量水溶液中的单盐和复盐S-BAIL的pH值。提出了单盐和复盐S-BAIL在水溶液中的离解机理。基于解离机理,提出了预测单盐和复盐S-BAILs pH值的方法,计算出的pH值与实验结果吻合良好。通过离子液体的离解率进一步解释了S-BAILs在水溶液中的离解机理,提出了一种估算稀水溶液中S-BAILs离解率的方法。
更新日期:2021-05-13
中文翻译:

单盐和双盐SO 3 H-官能化布朗斯台德酸性离子液体的pH值
酸性离子液体被广泛用作酸催化反应中的催化剂。SO 3的酸度H官能化的布朗斯台德酸性离子液体(S-BAILs)在水溶液中的pH值得到了系统的研究。用pH计测量水溶液中的单盐和复盐S-BAIL的pH值。提出了单盐和复盐S-BAIL在水溶液中的离解机理。基于解离机理,提出了预测单盐和复盐S-BAILs pH值的方法,计算出的pH值与实验结果吻合良好。通过离子液体的离解率进一步解释了S-BAILs在水溶液中的离解机理,提出了一种估算稀水溶液中S-BAILs离解率的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号