当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stem cells for bronchopulmonary dysplasia in preterm infants: A randomized controlled phase II trial
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2021-04-20 , DOI: 10.1002/sctm.20-0330 So Yoon Ahn 1 , Yun Sil Chang 1 , Myung Hee Lee 2 , Se In Sung 1 , Byong Sop Lee 3 , Ki Soo Kim 3 , Ai-Rhan Kim 3 , Won Soon Park 1
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2021-04-20 , DOI: 10.1002/sctm.20-0330 So Yoon Ahn 1 , Yun Sil Chang 1 , Myung Hee Lee 2 , Se In Sung 1 , Byong Sop Lee 3 , Ki Soo Kim 3 , Ai-Rhan Kim 3 , Won Soon Park 1
Affiliation
|
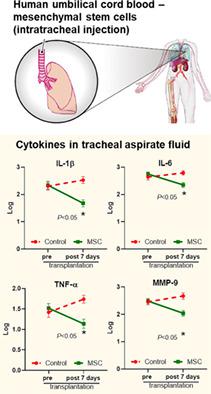
We previously demonstrated the safety and feasibility of mesenchymal stem cell (MSC) transplantation for bronchopulmonary dysplasia (BPD) in preterm infants in a phase I clinical trial. We thus investigated the therapeutic efficacy of MSCs for BPD in premature infants. A phase II double-blind, randomized, placebo-controlled clinical trial was conducted on preterm infants at 23 to 28 gestational weeks (GW) receiving mechanical ventilator support with respiratory deterioration between postnatal days 5 and 14. Infants were stratified by 23 to 24 GW and 25 to 28 GW and randomly allocated (1:1) to receive stem cells (1 × 107 cells/kg, n = 33) or placebo (n = 33). Although the inflammatory cytokines in the tracheal aspirate fluid were significantly reduced with MSCs, the primary outcome of death or severe/moderate BPD in the control group (18/33, 55%) was not significantly improved with MSC transplantation (17/33, 52%). In the subgroup analysis, the secondary outcome of severe BPD was significantly improved from 53% (8/15) to 19% (3/16) with MSC transplantation in the 23 to 24 GW group but not in the 25 to 28 GW subgroup. In summary, although MSC transplantation might be safe and feasible, this small study was underpowered to detect its therapeutic efficacy in preterm infants at 23 to 28 GW. Accordingly, we are now conducting an additional larger and controlled phase II clinical trial focusing on infants at 23 to 24 GW (NCT03392467). ClinicalTrials.gov identifier: NCT01828957.
中文翻译:

干细胞治疗早产儿支气管肺发育不良:一项随机对照 II 期试验
我们之前在 I 期临床试验中证明了间充质干细胞 (MSC) 移植治疗早产儿支气管肺发育不良 (BPD) 的安全性和可行性。因此,我们研究了 MSCs 对早产儿 BPD 的治疗效果。一项 II 期双盲、随机、安慰剂对照临床试验对 23 至 28 孕周 (GW) 接受机械呼吸机支持且在出生后第 5 天至第 14 天呼吸恶化的早产儿进行了分层。婴儿按 23 至 24 GW 分层和 25 到 28 GW 并随机分配 (1:1) 接收干细胞 (1 × 10 7细胞/kg,n = 33)或安慰剂(n = 33)。尽管 MSCs 显着降低了气管抽吸液中的炎性细胞因子,但对照组(18/33, 55%)死亡或重度/中度 BPD 的主要结果在 MSC 移植后没有显着改善(17/33, 52 %)。在亚组分析中,MSC 移植在 23 至 24 GW 组中,但在 25 至 28 GW 亚组中,严重 BPD 的次要结果从 53% (8/15) 显着改善至 19% (3/16)。总之,尽管 MSC 移植可能是安全可行的,但这项小型研究不足以检测其对 23 至 28 GW 早产儿的治疗效果。因此,我们现在正在进行一项更大的、受控的 II 期临床试验,重点是 23 至 24 GW 的婴儿 (NCT03392467)。ClinicalTrials.gov 标识符:
更新日期:2021-04-20
中文翻译:

干细胞治疗早产儿支气管肺发育不良:一项随机对照 II 期试验
我们之前在 I 期临床试验中证明了间充质干细胞 (MSC) 移植治疗早产儿支气管肺发育不良 (BPD) 的安全性和可行性。因此,我们研究了 MSCs 对早产儿 BPD 的治疗效果。一项 II 期双盲、随机、安慰剂对照临床试验对 23 至 28 孕周 (GW) 接受机械呼吸机支持且在出生后第 5 天至第 14 天呼吸恶化的早产儿进行了分层。婴儿按 23 至 24 GW 分层和 25 到 28 GW 并随机分配 (1:1) 接收干细胞 (1 × 10 7细胞/kg,n = 33)或安慰剂(n = 33)。尽管 MSCs 显着降低了气管抽吸液中的炎性细胞因子,但对照组(18/33, 55%)死亡或重度/中度 BPD 的主要结果在 MSC 移植后没有显着改善(17/33, 52 %)。在亚组分析中,MSC 移植在 23 至 24 GW 组中,但在 25 至 28 GW 亚组中,严重 BPD 的次要结果从 53% (8/15) 显着改善至 19% (3/16)。总之,尽管 MSC 移植可能是安全可行的,但这项小型研究不足以检测其对 23 至 28 GW 早产儿的治疗效果。因此,我们现在正在进行一项更大的、受控的 II 期临床试验,重点是 23 至 24 GW 的婴儿 (NCT03392467)。ClinicalTrials.gov 标识符:











































 京公网安备 11010802027423号
京公网安备 11010802027423号