Letters in Drug Design & Discovery ( IF 1 ) Pub Date : 2021-02-28 , DOI: 10.2174/1570180817999201005200505 Majid Ali 1 , Syed Majid Bukhari 2 , Asma Zaidi 2 , Farhan A. Khan 2 , Umer Rashid 2 , Neelum Tahir 2 , Baseerat Rabbani 2 , Umar Farooq 2
|
Background: Structurally diverse organic compounds and available drugs were screened against urease and carbonic anhydrase II in a formulation acceptable for high-throughput screening.
Objective: The study was conducted to find out potential inhibitors of urease and carbonic anhydrase II. Methods: Quantification of the possible HITs was carried out by determining their IC50 values.
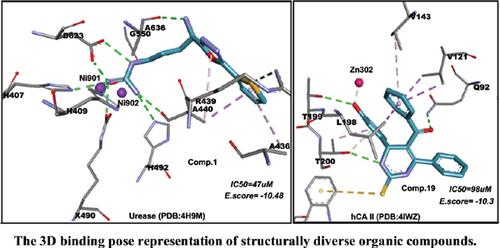
Results: The results of several screened compounds, including derivatives of oxadiazole, coumarins, chromane-2, 4-diones and metal complexes of cysteine-omeprazole showed promising inhibitory activities with IC50 ranging from 47 μM to 412 μM against the urease. The interactions of active compounds with active sites of enzymes were investigated through molecular docking studies which revealed that (R)-1-(4-amino-4-(5-(thiophen-2-yl)-1,3,4-oxadiazol-2-yl) butyl) guanidine possessing IC50 of 47 μM interacts with one of the nickel metal atoms of urease besides further interactions as predictable hydrogen bonds with KCX490, Asp633, His492, His407 and His409 along with Ala440 and 636. Bi-ligand metal complexes of 4-aminoantipyrine based Schiff bases showed activation of urease with AC50 ranging from 68 μM to 112 μM. Almost 21 compounds with varying functional groups including pyrimidines, oxadiazoles, imidazoles, hydrazides and tin based compounds were active carbonic anhydrase II inhibitors presenting 98 μM to 390 μM IC50 values. Several N-substituted sulfonamide derivatives were inactive against carbonic anhydrase II.
Conclusion: Among all the screened compounds, the highly active inhibitor of carbonic anhydrase II was (4-(3-hydroxyphenyl)-6-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl)phenyl) methanone with IC50 of 98.0 μM. This particular compound showed metallic interaction with Zn ion of carbonic anhydrase II through the hydroxyl group of the phenyl ring.
中文翻译:

高通量筛选和结构多样的有机化合物的分子对接研究对脲酶和碳酸酐酶II的抑制谱
背景:针对高通量筛选可接受的制剂,针对脲酶和碳酸酐酶II筛选了结构多样的有机化合物和可用药物。
目的:进行研究以寻找潜在的脲酶和碳酸酐酶II抑制剂。方法:通过确定IC 50值来量化可能的HIT 。
结果:几种筛选化合物的结果,包括恶二唑,香豆素,苯并二氢吡喃,4-二酮和半胱氨酸-奥美拉唑的金属配合物的衍生物,显示出有希望的抑制活性,对尿素酶的IC 50范围为47μM至412μM 。通过分子对接研究研究了活性化合物与酶活性位点之间的相互作用,结果表明(R)-1-(4-氨基-4-(5-(噻吩-2-基)-1,3,4-恶二唑)具有50μMIC 50的-2-yl)丁基)胍与脲酶的镍金属原子之一相互作用,此外还与KCX490,Asp633,His492,His407和His409以及Ala440和636形成可预测的氢键,从而进一步相互作用。基于4-氨基安替比林的席夫碱的金属配合物显示尿素酶被AC活化50的范围从68μM到112μM。几乎所有具有不同官能团的化合物(包括嘧啶,恶二唑,咪唑,酰肼和锡基化合物)都是活性碳酸酐酶II抑制剂,其IC 50值为98μM至390μM 。几种N-取代的磺酰胺衍生物对碳酸酐酶II无活性。
结论:在所有筛选的化合物中,碳酸酐酶II的高活性抑制剂为(4-(3-羟苯基)-6-苯基-2-硫代-1,2,3,4-四氢嘧啶-5-基)苯基。甲酮,IC 50为98.0μM。该特定化合物显示出通过苯环的羟基与碳酸酐酶II的Zn离子发生金属相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号