Chemical Geology ( IF 3.6 ) Pub Date : 2021-04-19 , DOI: 10.1016/j.chemgeo.2021.120258 Guojun Chen , Wenqi Zhao , Yang Yang , Dandan Chen , Ying Wang , Fangbai Li , Zhuyu Zhao , Fang Cao , Tongxu Liu
|
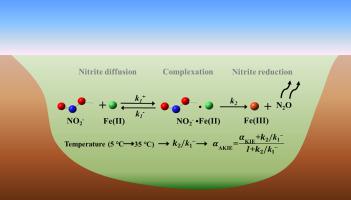
Chemodenitrification between Fe(II) and nitrite, an important nitrogen (N) cycling pathway, varies depending on the season, indicating the impact of ambient temperatures. However, the underlying mechanism for this occurrence has not been explained at a molecular scale. As an effective approach for studying N cycling, dual N O isotope fractionation was analyzed to reveal the reactions of temperature-dependent chemodenitrification. The kinetics of chemodenitrification under different temperatures showed that nitrite reduction rates and N2O production increased with temperature (5–35 °C). Lepidocrocite was identified as the secondary mineral of Fe(II) oxidation at 5, 15, and 25 °C, and goethite was observed at 35 °C. Further, the value of the O isotope enrichment factor (18ε) decreased with increasing temperature while values of N isotope fractionation enrichment factor (15ε) were less sensitive to temperature. The isotope fractionation ratios of O to N (18ε:15ε) were 1.00 ± 0.25, 0.75 ± 0.05, 0.54 ± 0.04, and 0.46 ± 0.06 at 5, 15, 25, and 35 °C, respectively, indicating that the temperature effect should be considered when dual N
O isotope fractionation was analyzed to reveal the reactions of temperature-dependent chemodenitrification. The kinetics of chemodenitrification under different temperatures showed that nitrite reduction rates and N2O production increased with temperature (5–35 °C). Lepidocrocite was identified as the secondary mineral of Fe(II) oxidation at 5, 15, and 25 °C, and goethite was observed at 35 °C. Further, the value of the O isotope enrichment factor (18ε) decreased with increasing temperature while values of N isotope fractionation enrichment factor (15ε) were less sensitive to temperature. The isotope fractionation ratios of O to N (18ε:15ε) were 1.00 ± 0.25, 0.75 ± 0.05, 0.54 ± 0.04, and 0.46 ± 0.06 at 5, 15, 25, and 35 °C, respectively, indicating that the temperature effect should be considered when dual N O isotope data are applied to distinguish chemodenitrification with biodenitrification . A kinetic model was established to correlate the rate constants of the elementary reactions during chemodenitrification with N
O isotope data are applied to distinguish chemodenitrification with biodenitrification . A kinetic model was established to correlate the rate constants of the elementary reactions during chemodenitrification with N O isotope fractionation. The intrinsic isotope fractionation of N
O isotope fractionation. The intrinsic isotope fractionation of N O bond breakage during chemodenitrification was obtained. This study provides both qualitative and quantitative outcomes on temperature-dependent chemodenitrification. This study provides inputs for a better understanding of the role of chemodenitrification in effecting seasonal changes to the environment.
O bond breakage during chemodenitrification was obtained. This study provides both qualitative and quantitative outcomes on temperature-dependent chemodenitrification. This study provides inputs for a better understanding of the role of chemodenitrification in effecting seasonal changes to the environment.
中文翻译:

Fe(II)和亚硝酸盐的化学反硝化:温度和双重N
 O同位素分馏的影响
O同位素分馏的影响
Fe(II)和亚硝酸盐(一种重要的氮(N)循环途径)之间的化学氮化根据季节而变化,表明环境温度的影响。但是,这种发生的潜在机理尚未在分子水平上得到解释。作为研究氮循环的有效方法, 分析了双重氮同位素分馏以揭示温度依赖性化学反硝化反应。在不同温度下化学脱氮的动力学表明,亚硝酸盐的还原速率和N 2 O的产生随温度(5-35°C)的增加而增加。纤铁矿被鉴定为在5、15和25°C时Fe(II)氧化的次生矿物,在35°C时观察到针铁矿。另外,将O同位素富集因子的值(18 ε),同时Ñ同位素分馏富集因子(的值随温度升高而降低15 ε)为对温度较不敏感。的O的同位素分馏比率N(18 ε:15 ε)分别为1.00±0.25,0.75±0.05,0.54±0.04,并在5 0.46±0.06,15,25,和35℃,分别表明温度当使用双重N
分析了双重氮同位素分馏以揭示温度依赖性化学反硝化反应。在不同温度下化学脱氮的动力学表明,亚硝酸盐的还原速率和N 2 O的产生随温度(5-35°C)的增加而增加。纤铁矿被鉴定为在5、15和25°C时Fe(II)氧化的次生矿物,在35°C时观察到针铁矿。另外,将O同位素富集因子的值(18 ε),同时Ñ同位素分馏富集因子(的值随温度升高而降低15 ε)为对温度较不敏感。的O的同位素分馏比率N(18 ε:15 ε)分别为1.00±0.25,0.75±0.05,0.54±0.04,并在5 0.46±0.06,15,25,和35℃,分别表明温度当使用双重N  O同位素数据来区分化学脱氮和生物脱氮时,应考虑效果。建立了动力学模型,以将化学硝化过程中基本反应的速率常数与N
O同位素数据来区分化学脱氮和生物脱氮时,应考虑效果。建立了动力学模型,以将化学硝化过程中基本反应的速率常数与N  O同位素分馏相关联。N的固有同位素分馏
O同位素分馏相关联。N的固有同位素分馏 获得了化学硝化过程中的O键断裂。这项研究提供了依赖温度的化学脱氮的定性和定量结果。这项研究为更好地了解化学氮化在影响环境季节性变化中的作用提供了投入。
获得了化学硝化过程中的O键断裂。这项研究提供了依赖温度的化学脱氮的定性和定量结果。这项研究为更好地了解化学氮化在影响环境季节性变化中的作用提供了投入。









































 京公网安备 11010802027423号
京公网安备 11010802027423号