当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Diagram and Thermodynamic Properties of the EuBr2–CsBr Binary System
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-04-19 , DOI: 10.1021/acs.jced.0c01016 Leszek Rycerz 1 , Jan Kapała 1 , Marcelle Gaune-Escard 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-04-19 , DOI: 10.1021/acs.jced.0c01016 Leszek Rycerz 1 , Jan Kapała 1 , Marcelle Gaune-Escard 2
Affiliation
|
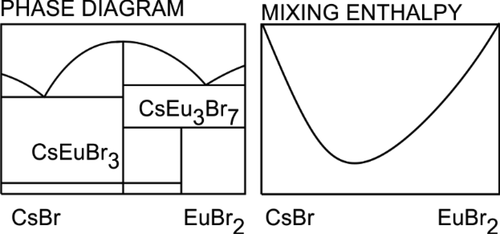
Phase equilibria in the EuBr2–CsBr binary system were investigated by the differential scanning calorimetry method. The mixing enthalpy of the liquid phase at 1055 K was measured using a Calvet calorimeter over the whole composition range. Two compounds, CsEuBr3, congruently melted at 1034 K, and CsEu3Br7, decomposed at 704 K, were found in the solid phase. Two eutectics were located at xEuBr2 = 0.197 (T = 812 K) and xEuBr2 = 0.821 (T = 864 K). The heat capacities of solid and liquid phases of CsEuBr3 were measured from 300 to 1100 K. The CALPHAD method was used to verify the thermodynamic phase compatibility of the system. The Gibbs free energy of formation of solid compounds was calculated.
中文翻译:

EuBr 2 -CsBr二元体系的相图和热力学性质
通过差示扫描量热法研究了EuBr 2 -CsBr二元体系中的相平衡。使用Calvet量热计在整个组成范围内测量在1055K下液相的混合焓。在固相中发现了两种化合物,CsEuBr 3在1034 K下完全熔化,CsEu 3 Br 7在704 K下分解。两个共晶位于x EuBr 2 = 0.197(T = 812 K)和x EuBr 2 = 0.821(T = 864 K)。CsEuBr 3的固相和液相的热容在300到1100 K范围内测量。CALPHAD方法用于验证系统的热力学相兼容性。计算了形成固体化合物的吉布斯自由能。
更新日期:2021-05-13
中文翻译:

EuBr 2 -CsBr二元体系的相图和热力学性质
通过差示扫描量热法研究了EuBr 2 -CsBr二元体系中的相平衡。使用Calvet量热计在整个组成范围内测量在1055K下液相的混合焓。在固相中发现了两种化合物,CsEuBr 3在1034 K下完全熔化,CsEu 3 Br 7在704 K下分解。两个共晶位于x EuBr 2 = 0.197(T = 812 K)和x EuBr 2 = 0.821(T = 864 K)。CsEuBr 3的固相和液相的热容在300到1100 K范围内测量。CALPHAD方法用于验证系统的热力学相兼容性。计算了形成固体化合物的吉布斯自由能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号