Environmental Technology & Innovation ( IF 6.7 ) Pub Date : 2021-04-18 , DOI: 10.1016/j.eti.2021.101559 Peyman Koohi , Ahmad Rahbar-kelishami , Hadi Shayesteh
|
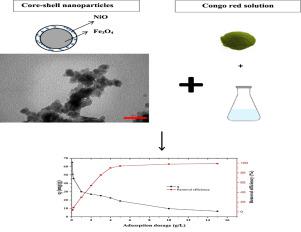
In the present study, the adsorption of congo red (CR) using Fe3O4/NiO magnetic nanocomposites synthesized via a precipitation method was studied. XRD, EDS, FTIR, TEM, BET, and SEM techniques were used to obtain their specifications. The influence of solution pH, adsorbent dosage, time of experiments, initial concentration of CR, and the temperature has been checked. Among the non-linear isotherm models studied, Langmuir with mg/g at 293 K and showed the highest agreement with laboratory results compared to Freundlich, Jovanovic, Halsey, and Temkin models. The results also showed the congo red’s adsorption process on Fe3O4/NiO nanocomposite follows the pseudo-second-order kinetic model. Further, temperature and thermodynamic studies’ effect was carried out, and results showed spontaneously and exothermically the adsorption process. Overall, Fe3O4/NiO nanocomposite, due to low equilibrium time and high adsorption capacity, could remove congo red from contaminated water.
中文翻译:

用铁有效去除刚果红染料Ø/ NiO纳米复合材料:合成与表征
在本研究中,研究了通过沉淀法合成的Fe 3 O 4 / NiO磁性纳米复合材料对刚果红(CR)的吸附。使用XRD,EDS,FTIR,TEM,BET和SEM技术获得其规格。已经检查了溶液pH,吸附剂用量,实验时间,CR的初始浓度和温度的影响。在研究的非线性等温线模型中,Langmuir与 293 K下的mg / g和 与Freundlich,Jovanovic,Halsey和Temkin模型相比,实验结果的一致性最高。结果还表明刚果红在Fe 3 O 4 / NiO纳米复合材料上的吸附过程遵循拟二级动力学模型。此外,进行了温度和热力学研究,结果自发地和放热地表明了吸附过程。总的来说,Fe 3 O 4 / NiO纳米复合材料由于平衡时间短和吸附能力强,可以从受污染的水中去除刚果红。











































 京公网安备 11010802027423号
京公网安备 11010802027423号