当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inducing non-stoichiometry in nano-hydroxyapatite for ultra-fast sequestration of uranyl ions in water: mechanism delineation using XAS
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2021-3-30 , DOI: 10.1039/d0en01246g Sabyasachi Rout 1, 2, 3, 4 , Nitin Khandelwal 4, 5, 6, 7 , A. K. Poswal 2, 3, 4, 8 , Vandana Pulhani 1, 2, 3, 4 , A. V. Kumar 1, 2, 3, 4
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2021-3-30 , DOI: 10.1039/d0en01246g Sabyasachi Rout 1, 2, 3, 4 , Nitin Khandelwal 4, 5, 6, 7 , A. K. Poswal 2, 3, 4, 8 , Vandana Pulhani 1, 2, 3, 4 , A. V. Kumar 1, 2, 3, 4
Affiliation
|
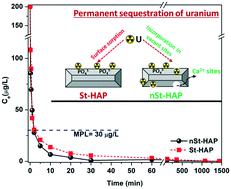
The presence of radionuclides in water bodies imposes severe environmental concerns, and thus their permanent sequestration is of immense importance. In this study, the sorption of uranyl ions was comparatively evaluated using as-synthesized stoichiometric and non-stoichiometric hydroxyapatite (St-HAP and nSt-HAP, respectively) nanomaterials. Results showed the successful synthesis of both St-HAP (Ca/P = 1.67) and nSt-HAP (Ca/P = 1.35), which possessed a nanorod-like structure. The sorption studies confirmed the efficient and ultrafast removal of uranium. The induction of non-stoichiometry resulted in comparatively faster removal, i.e., below the drinking water permissible limit within 2 min of interaction, with an elevated sorption capacity of 175 mg g−1 compared to 97 mg g−1 of St-HAP. The impact of all the studied environmental parameters such as pH, ionic strength, bicarbonate and humic acid was found to be minimal for nSt-HAP, suggesting the selective removal of uranium with good environmental applicability of nSt-HAP. Due to the higher uranium sorption capacity of the Ca-deficient nSt-HAP, the mechanism of sorption was investigated in terms of the bonding state using the XAS technique. This study revealed that specific sites are available where U(VI) is preferentially trapped in the case of nSt-HAP, which led to the enhanced and ultrafast sequestration of uranium from water.
中文翻译:

诱导纳米羟基磷灰石中非化学计量的超快螯合水中铀酰离子:使用XAS的机理描述
水体中放射性核素的存在引起了严重的环境问题,因此将其永久隔离至关重要。在这项研究中,使用合成的化学计量和非化学计量的羟基磷灰石(分别为St-HAP和nSt-HAP)纳米材料对铀酰离子的吸附进行了比较评估。结果表明,具有纳米棒状结构的St-HAP(Ca / P = 1.67)和nSt-HAP(Ca / P = 1.35)均成功合成。吸附研究证实了铀的有效和超快速去除。非化学计量的诱导导致相对较快的去除,即在相互作用后2分钟内达到饮用水允许极限以下,与97 mg g相比,吸附能力提高了175 mg g -1St-HAP的-1。已发现所有研究的环境参数(例如pH,离子强度,碳酸氢盐和腐殖酸)对nSt-HAP的影响极小,这表明选择性去除铀对nSt-HAP具有良好的环境适用性。由于缺钙的nSt-HAP的铀吸附能力更高,因此使用XAS技术根据键合状态研究了吸附机理。这项研究表明,在nSt-HAP的情况下,可以优先捕获U( VI)的特定位点,从而导致铀从水中的富集和超快螯合。
更新日期:2021-04-16
中文翻译:

诱导纳米羟基磷灰石中非化学计量的超快螯合水中铀酰离子:使用XAS的机理描述
水体中放射性核素的存在引起了严重的环境问题,因此将其永久隔离至关重要。在这项研究中,使用合成的化学计量和非化学计量的羟基磷灰石(分别为St-HAP和nSt-HAP)纳米材料对铀酰离子的吸附进行了比较评估。结果表明,具有纳米棒状结构的St-HAP(Ca / P = 1.67)和nSt-HAP(Ca / P = 1.35)均成功合成。吸附研究证实了铀的有效和超快速去除。非化学计量的诱导导致相对较快的去除,即在相互作用后2分钟内达到饮用水允许极限以下,与97 mg g相比,吸附能力提高了175 mg g -1St-HAP的-1。已发现所有研究的环境参数(例如pH,离子强度,碳酸氢盐和腐殖酸)对nSt-HAP的影响极小,这表明选择性去除铀对nSt-HAP具有良好的环境适用性。由于缺钙的nSt-HAP的铀吸附能力更高,因此使用XAS技术根据键合状态研究了吸附机理。这项研究表明,在nSt-HAP的情况下,可以优先捕获U( VI)的特定位点,从而导致铀从水中的富集和超快螯合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号