当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alternate pathway for standard SCR on Cu-zeolites with gas-phase ammonia
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2021-4-13 , DOI: 10.1039/d1re00041a Rohil Daya 1, 2, 3 , Christopher J. Keturakis 3, 4, 5 , Dylan Trandal 1, 2, 3 , Ashok Kumar 1, 2, 3 , Saurabh Y. Joshi 1, 2, 3 , Aleksey Yezerets 1, 2, 3
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2021-4-13 , DOI: 10.1039/d1re00041a Rohil Daya 1, 2, 3 , Christopher J. Keturakis 3, 4, 5 , Dylan Trandal 1, 2, 3 , Ashok Kumar 1, 2, 3 , Saurabh Y. Joshi 1, 2, 3 , Aleksey Yezerets 1, 2, 3
Affiliation
|
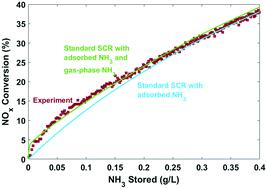
Redox mechanisms have been theorized for the selective catalytic reduction (SCR) of NOx over small-pore Cu-zeolites. These mechanisms generally rely on NH3 solvation of active copper sites as the initial step in the reduction half cycle for standard SCR. In this work, we demonstrate that this pre-requisite for NH3 solvation of active copper sites is inconsistent with experimental data for initial NO consumption under SCR conditions. Reactor data under standard SCR and reduction half cycle (no O2) conditions shows instantaneous consumption of NO upon introduction of NO and NH3. However, detailed and global kinetic models relying on a sequential adsorption–conversion mechanism predict an initial delay in NO consumption associated with the relatively slow macroscopic coverage dynamics. These model discrepancies at low NH3 coverage are quantitatively resolved by relaxing the pre-requisite of NH3 storage for NOx conversion, via an alternate SCR pathway utilizing gas-phase NH3. Addition of this standard SCR reaction via gas-phase NH3 leads to predicted NH3 reaction orders >1 at low NH3 coverage, confirmed subsequently by experiments. The results shown here are consistent with an energetically feasible mechanistic pathway involving the reduction of copper sites by NO to generate mobile HONO intermediates, which can potentially react with gas-phase NH3 in the zeolite cages.
中文翻译:

含氨的铜沸石上标准SCR的替代途径
氧化还原机制已经被理论化的选择性催化还原(SCR)的NO X在小孔的Cu-沸石。这些机制通常依赖于活性铜位点的NH 3溶剂化,作为标准SCR还原半循环的第一步。在这项工作中,我们证明了活性铜位点NH 3溶剂化的先决条件与SCR条件下初始NO消耗的实验数据不一致。在标准SCR和还原半循环(无O 2)条件下的反应器数据显示,引入NO和NH 3后瞬时消耗NO。然而,依赖于顺序吸附-转化机制的详细的整体动力学模型预测,与相对较慢的宏观覆盖动力学有关的NO消耗会出现初始延迟。在低NH 3覆盖率下,这些模型差异可通过使用气相NH 3的备用SCR途径,通过放宽NH 3储存进行NO x转化的先决条件来定量解决。通过气相NH 3添加此标准SCR反应可导致在低NH 3下预测的NH 3反应阶数> 1覆盖率,随后通过实验确认。此处显示的结果与在能量上可行的机制途径一致,该途径涉及通过NO还原铜位以生成可移动的HONO中间体,该中间体可能与沸石笼中的气相NH 3反应。
更新日期:2021-04-16
中文翻译:

含氨的铜沸石上标准SCR的替代途径
氧化还原机制已经被理论化的选择性催化还原(SCR)的NO X在小孔的Cu-沸石。这些机制通常依赖于活性铜位点的NH 3溶剂化,作为标准SCR还原半循环的第一步。在这项工作中,我们证明了活性铜位点NH 3溶剂化的先决条件与SCR条件下初始NO消耗的实验数据不一致。在标准SCR和还原半循环(无O 2)条件下的反应器数据显示,引入NO和NH 3后瞬时消耗NO。然而,依赖于顺序吸附-转化机制的详细的整体动力学模型预测,与相对较慢的宏观覆盖动力学有关的NO消耗会出现初始延迟。在低NH 3覆盖率下,这些模型差异可通过使用气相NH 3的备用SCR途径,通过放宽NH 3储存进行NO x转化的先决条件来定量解决。通过气相NH 3添加此标准SCR反应可导致在低NH 3下预测的NH 3反应阶数> 1覆盖率,随后通过实验确认。此处显示的结果与在能量上可行的机制途径一致,该途径涉及通过NO还原铜位以生成可移动的HONO中间体,该中间体可能与沸石笼中的气相NH 3反应。







































 京公网安备 11010802027423号
京公网安备 11010802027423号