当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A time-dependent density function theory study on the substituent effect on excited-state intramolecular proton transfer of 4′-methoxy-3-hydroxyl flavone
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2021-04-16 , DOI: 10.1002/poc.4216 Mei Ni 1 , Xiuning Liang 1 , Hua Fang 1
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2021-04-16 , DOI: 10.1002/poc.4216 Mei Ni 1 , Xiuning Liang 1 , Hua Fang 1
Affiliation
|
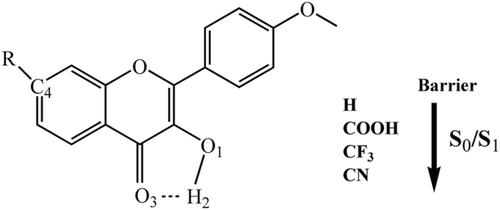
Theoretical investigations on the excited-state intramolecular proton transfer (ESIPT) in the 4′-methoxy-3-hydroxyl flavone (MHF) derivatives 4R-MHF (R: COOH, CF3, and CN) are performed based on time-dependent density function theory (TD-DFT). According to our calculations, the intramolecular H bondings in 4R-MHF (R: COOH, CF3, and CN) are weakened by the substituents, which would be unfavorable to ESIPT reaction. ESIPT processes in the 4R-MHF (R: COOH, CF3, and CN) are slightly harder than that in MHF because the ESIPT barriers of 4R-MHF (R: COOH, CF3, and CN) are on average 0.38 kcal/mol larger than that of MHF. The stronger the electron-withdrawing ability, the larger is the ESIPT potential barrier.
中文翻译:

4'-甲氧基-3-羟基黄酮激发态分子内质子转移的取代基效应的瞬态密度函数理论研究
基于时间依赖性密度对 4'-甲氧基-3-羟基黄酮 (MHF) 衍生物 4R-MHF(R: COOH、CF 3和 CN)中的激发态分子内质子转移 (ESIPT)进行理论研究函数论(TD-DFT)。根据我们的计算,4R-MHF(R:COOH、CF 3和CN)中的分子内H键被取代基削弱,这不利于ESIPT反应。4R-MHF(R:COOH、CF 3和 CN)中的 ESIPT 过程比 MHF 中的稍难,因为 4R-MHF(R:COOH、CF 3和 CN)的 ESIPT 势垒平均为 0.38 kcal/ mol 比 MHF 大。吸电子能力越强,ESIPT 势垒越大。
更新日期:2021-04-16
中文翻译:

4'-甲氧基-3-羟基黄酮激发态分子内质子转移的取代基效应的瞬态密度函数理论研究
基于时间依赖性密度对 4'-甲氧基-3-羟基黄酮 (MHF) 衍生物 4R-MHF(R: COOH、CF 3和 CN)中的激发态分子内质子转移 (ESIPT)进行理论研究函数论(TD-DFT)。根据我们的计算,4R-MHF(R:COOH、CF 3和CN)中的分子内H键被取代基削弱,这不利于ESIPT反应。4R-MHF(R:COOH、CF 3和 CN)中的 ESIPT 过程比 MHF 中的稍难,因为 4R-MHF(R:COOH、CF 3和 CN)的 ESIPT 势垒平均为 0.38 kcal/ mol 比 MHF 大。吸电子能力越强,ESIPT 势垒越大。



























 京公网安备 11010802027423号
京公网安备 11010802027423号