当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Salinity on Hydrate Phase Equilibrium and Kinetics of SF6, HFC134a, and Their Mixture
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-04-16 , DOI: 10.1021/acs.jced.1c00139 Hai Son Truong-Lam 1, 2 , Seungmin Lee 1 , Changsu Jeon 1 , SeongDeok Seo 1 , Kyung Chan Kang 1 , Ju Dong Lee 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-04-16 , DOI: 10.1021/acs.jced.1c00139 Hai Son Truong-Lam 1, 2 , Seungmin Lee 1 , Changsu Jeon 1 , SeongDeok Seo 1 , Kyung Chan Kang 1 , Ju Dong Lee 1
Affiliation
|
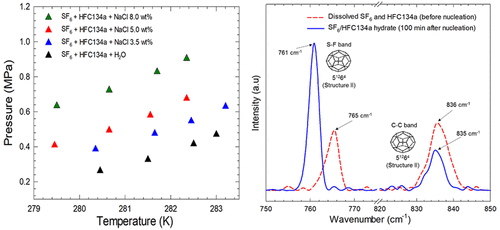
In this study, the effects of salinity on the equilibrium and kinetics of sulfur hexafluoride (SF6), 1,1,1,2-tetrafluoroethane (HFC134a), and their mixture were evaluated to analyze their potential applications to hydrate-based desalination. The equilibrium pressure of the mixture gas (SF6/HFC134a) was lower than that of pure SF6 gas but higher than that of pure HFC134a gas. The thermodynamic effects of various concentrations (0, 3.5, 5, and 8 wt %) of NaCl on the gas hydrates were evaluated. Experiments were performed on the formation kinetics in the presence of NaCl solution at the same experimental temperature and pressure (274.15 K and 0.70 MPa). The hydrate growth rates decreased with increasing NaCl concentration. The rates also declined with time, associated with the inhibition of mass transfer between the gas and the liquid. During hydrate formation, the vapor compositions of the mixture gas were analyzed by in situ Raman spectroscopy and the results were consistent with those obtained by gas chromatography. Furthermore, an in situ Raman spectroscopic analysis demonstrated that the SF6 and HFC134a molecules occupy only the large cage (51264) of the structure II (sII) hydrate during SF6/HFC134a formation and the addition of salt did not change the structure of the SF6 and HFC134a hydrates. The hydrate phase behavior and kinetic results of this study can serve as foundational data for hydrate-based desalination technology and fluorinated gases separation and recovery processes.
中文翻译:

盐度对SF 6,HFC134a及其混合物水合相平衡和动力学的影响
在这项研究中,盐度对六氟化硫(SF 6),1,1,1,2-四氟乙烷(HFC134a)及其混合物的平衡和动力学的影响进行了评估,以分析其在基于水合物的脱盐中的潜在应用。混合气体(SF 6 / HFC134a)的平衡压力低于纯SF 6的平衡压力气体,但高于纯HFC134a气体。评估了各种浓度(0、3.5、5和8 wt%)的NaCl对气体水合物的热力学效应。在相同的实验温度和压力(274.15 K和0.70 MPa)下,在存在NaCl溶液的条件下对形成动力学进行了实验。水合物的生长速率随NaCl浓度的增加而降低。速率也随着时间下降,这与气体和液体之间的质量转移受到抑制有关。在水合物形成期间,通过原位拉曼光谱法分析了混合气体的蒸气组成,其结果与通过气相色谱法获得的结果一致。此外,原位拉曼光谱分析表明,SF 6HSF134a和HFC134a分子仅在SF 6 / HFC134a形成过程中占据了结构II(sII)水合物的大笼子(5 12 6 4),盐的添加并未改变SF 6和HFC134a水合物的结构。该研究的水合物相行为和动力学结果可为基于水合物的脱盐技术以及氟化气体的分离和回收过程提供基础数据。
更新日期:2021-05-13
中文翻译:

盐度对SF 6,HFC134a及其混合物水合相平衡和动力学的影响
在这项研究中,盐度对六氟化硫(SF 6),1,1,1,2-四氟乙烷(HFC134a)及其混合物的平衡和动力学的影响进行了评估,以分析其在基于水合物的脱盐中的潜在应用。混合气体(SF 6 / HFC134a)的平衡压力低于纯SF 6的平衡压力气体,但高于纯HFC134a气体。评估了各种浓度(0、3.5、5和8 wt%)的NaCl对气体水合物的热力学效应。在相同的实验温度和压力(274.15 K和0.70 MPa)下,在存在NaCl溶液的条件下对形成动力学进行了实验。水合物的生长速率随NaCl浓度的增加而降低。速率也随着时间下降,这与气体和液体之间的质量转移受到抑制有关。在水合物形成期间,通过原位拉曼光谱法分析了混合气体的蒸气组成,其结果与通过气相色谱法获得的结果一致。此外,原位拉曼光谱分析表明,SF 6HSF134a和HFC134a分子仅在SF 6 / HFC134a形成过程中占据了结构II(sII)水合物的大笼子(5 12 6 4),盐的添加并未改变SF 6和HFC134a水合物的结构。该研究的水合物相行为和动力学结果可为基于水合物的脱盐技术以及氟化气体的分离和回收过程提供基础数据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号