Water Research ( IF 11.4 ) Pub Date : 2021-04-15 , DOI: 10.1016/j.watres.2021.117143 Chu Yingying , Xu Lijie , Gan Lu , Qiao Weichuan , Han Jiangang , Mei Xiang , Guo He , Li Wei , Pei Chun , Gong Han , Guo Xuewen
|
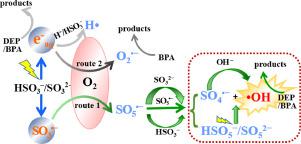
UV/sulfite systems with oxygen have recently been considered as advanced oxidation processes in view of the participation of oxysulfur radicals. However, the contribution of •OH and the efficiency of destructing emerging contaminants (ECs) in water remain largely unclear. Here, the UV/S(IV) process was applied with natural reoxygenation to degrade two typical ECs, diethyl phthalate (DEP) and bisphenol A (BPA) showing different properties. Solution pH played the key role in determining the reactive species, and both DEP and BPA were more favorably degraded at more alkaline conditions with higher utilization efficiency of SO32−. Specifically, the H•, O2•−, •OH and SO3•− were identified at acidic condition, but the amount of •OH accumulated significantly with the elevation of pH. Competitive quenching experiments showed that eaq− and •OH dominated the degradation of DEP and BPA at alkaline condition, respectively. Besides, DEP showed higher quantum efficiency for the indirect photolysis and mineralization degree than that of BPA at pH 9.2 mainly due to the direct use of the primary photoproduct. The possible transformation mechanisms of S(IV) and mineralization routes of both pollutants were proposed. This study may provide new insights into the mechanisms involved in UV/S(IV) process and a promising alternative for efficient removal of ECs in water.
中文翻译:

通过自然复氧的UV / S(IV)工艺有效破坏水中的新兴污染物:pH对反应物种的影响
考虑到氧-硫自由基的参与,具有氧气的UV /亚硫酸盐体系最近被认为是高级氧化方法。但是,•OH的贡献和破坏水中新兴污染物(EC)的效率仍不清楚。在这里,UV / S(IV)工艺经过自然再氧化处理,以降解两种典型的EC,邻苯二甲酸二乙酯(DEP)和双酚A(BPA)显示出不同的性能。溶液的pH值在确定反应性物种中起着关键作用,DEP和BPA在更碱性的条件下均能更有利地降解,并具有更高的SO 3 2-利用率。具体而言,H•,O 2 •-,•OH和SO 3 •-可以在酸性条件下进行鉴定,但是•OH的含量随pH的升高而显着积累。竞争淬火实验表明,电子含水-和•OH分别为主DEP和BPA的降解在碱性条件下。此外,DEP在pH 9.2时对间接光解和矿化度的量子效率比BPA高,这主要是由于直接使用了初级光产物。提出了两种污染物可能的S(IV)转化机理和矿化途径。这项研究可能会提供有关UV / S(IV)过程中涉及的机制的新见解,以及有效去除水中EC的有前途的替代方法。









































 京公网安备 11010802027423号
京公网安备 11010802027423号