当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
o-Nitroacetanilide Equilibrium Solubility in 15 Monosolvents: Experimental Determination, Mathematical Correlation, and Solvent Effect Examination
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-04-13 , DOI: 10.1021/acs.jced.1c00037 Hongkun Zhao 1 , Yuxin Bao 1 , Ali Farajtabar 2 , Abolghasem Jouyban 3 , William E. Acree 4
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-04-13 , DOI: 10.1021/acs.jced.1c00037 Hongkun Zhao 1 , Yuxin Bao 1 , Ali Farajtabar 2 , Abolghasem Jouyban 3 , William E. Acree 4
Affiliation
|
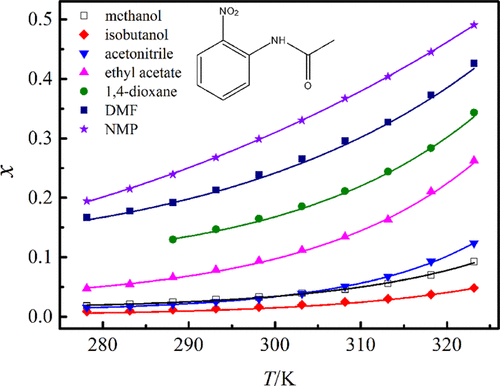
Equilibrium solubility of o-nitroacetanilide in 15 monosolvents (ethylene glycol, n-propanol, methanol, ethyl acetate, N,N-dimethylformamide, ethanol, isopropanol, acetonitrile, 1,4-dioxane, n-butanol, isobutanol, n-heptanol, water, cyclohexane, and N-methyl pyrrolidone) was acquired through experiments that were conducted by a shake-flask method under a local pressure of 101.2 kPa at elevated temperatures from 278.15 to 323.15 K. The decreasing tendency of solubility magnitudes in the 15 monosolvents was N-methyl pyrrolidone > N,N-dimethylformamide > 1,4-dioxane > ethyl acetate > methanol (acetonitrile) > ethanol > n-propanol > n-butanol > isobutanol > isopropanol > n-heptanol > ethylene glycol > cyclohexane > water. No polymorphic transformation or solvation phenomenon happened after equilibration in the 15 solvents. Solubility correlations were made through two semiempirical equations (Apelblat equation and λh equation) and two activity coefficient models (NRTL model and Wilson model). The maximum root-mean-square and relative average deviations acquired through back-calculation were 87.57 × 10–4 and 8.95%, respectively. The correlating result was better through Apelblat equation than through the other models and equations. Examination was made for the molecular interactions between solvent–solvent and solute–solvent species by the linear solvation energy relationships. In addition, this work reported the thermodynamic mixing properties, infinite dilution activity coefficient, and reduced excess enthalpy achieved through the Wilson model.
中文翻译:

在15种单溶剂中的邻硝基乙酰苯胺平衡溶解度:实验测定,数学相关性和溶剂作用检验
的平衡溶解度Ó在15个monosolvents -nitroacetanilide(乙二醇,Ñ丙醇,甲醇,乙酸乙酯,Ñ,Ñ二甲基甲酰胺,乙醇,异丙醇,乙腈,1,4-二恶烷,Ñ丁醇,异丁醇,Ñ庚醇,水,环己烷和N-甲基吡咯烷酮)是通过摇瓶法在101.2 kPa局部压力下在278.15至323.15 K的高温下进行的实验而获得的。在15种单溶剂中,溶解度的下降趋势为N-甲基吡咯烷酮> N,N-二甲基甲酰胺> 1,4-二恶烷>乙酸乙酯>甲醇(乙腈)>乙醇>正丙醇>正丁醇>异丁醇>异丙醇>正庚醇>乙二醇>环己烷>水。在15种溶剂中平衡后,未发生多晶型转变或溶剂化现象。溶解度相关性进行了通过两个半经验公式(Apelblat方程和λ制成ħ方程)和两个活度系数模型(NRTL模型和Wilson模型)。通过反向计算获得的最大均方根和相对平均偏差为87.57×10 –4和8.95%。通过Apelblat方程,相关结果比通过其他模型和方程更好。通过线性溶剂化能量关系对溶剂-溶剂和溶质-溶剂种类之间的分子相互作用进行了研究。此外,这项工作报告了通过威尔逊模型获得的热力学混合特性,无限稀释活性系数和减少的过量焓。
更新日期:2021-05-13
中文翻译:

在15种单溶剂中的邻硝基乙酰苯胺平衡溶解度:实验测定,数学相关性和溶剂作用检验
的平衡溶解度Ó在15个monosolvents -nitroacetanilide(乙二醇,Ñ丙醇,甲醇,乙酸乙酯,Ñ,Ñ二甲基甲酰胺,乙醇,异丙醇,乙腈,1,4-二恶烷,Ñ丁醇,异丁醇,Ñ庚醇,水,环己烷和N-甲基吡咯烷酮)是通过摇瓶法在101.2 kPa局部压力下在278.15至323.15 K的高温下进行的实验而获得的。在15种单溶剂中,溶解度的下降趋势为N-甲基吡咯烷酮> N,N-二甲基甲酰胺> 1,4-二恶烷>乙酸乙酯>甲醇(乙腈)>乙醇>正丙醇>正丁醇>异丁醇>异丙醇>正庚醇>乙二醇>环己烷>水。在15种溶剂中平衡后,未发生多晶型转变或溶剂化现象。溶解度相关性进行了通过两个半经验公式(Apelblat方程和λ制成ħ方程)和两个活度系数模型(NRTL模型和Wilson模型)。通过反向计算获得的最大均方根和相对平均偏差为87.57×10 –4和8.95%。通过Apelblat方程,相关结果比通过其他模型和方程更好。通过线性溶剂化能量关系对溶剂-溶剂和溶质-溶剂种类之间的分子相互作用进行了研究。此外,这项工作报告了通过威尔逊模型获得的热力学混合特性,无限稀释活性系数和减少的过量焓。











































 京公网安备 11010802027423号
京公网安备 11010802027423号