Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2021-04-14 , DOI: 10.1016/j.jece.2021.105503 Paulo Vitor Brandão Leal , Douglas Henrique Pereira , Rísia Magriotis Papini , Zuy Maria Magriotis
|
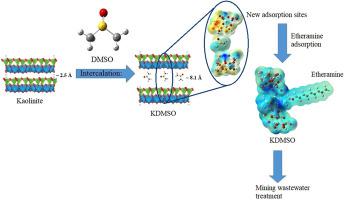
This study evaluated the effect of dimethyl sulfoxide (DMSO) intercalation in natural kaolinite (KN) on etheramine adsorption. The effects of the parameters pH, initial etheramine concentration and adsorbent mass were investigated using central composite design. Optimal conditions were determined using response surface methodology. Theoretical calculations were performed to optimize the geometries of the kaolinite, DMSO, KDMSO, molecular and protonated etheramine structures, as well as the interaction between etheramine and kaolinite. XRD and FTIR results confirm the intercalation of DMSO into kaolinite, the intercalation process caused a significant increase in the isoelectric point. The results show that the adsorption was more efficient in pH 10 and etheramine concentration of 400 mg L-1 for both adsorbents, and 0.1 g of KN and 0.2 g of KDMSO. The kinetic data most accurately fit the pseudo-second-order model. The fitting of the experimental data to the isotherm models indicated that the Sips is the most appropriate model. The calculation of Eads revealed that mechanism of etheramine removal by KN and KDMSO involve chemisorption. The reusability tests determined that after four uses, the etheramine removal efficiency does not change significantly, enabling the use of kaolinite for wastewater treatment. Theoretical studies have enabled a better understanding of the intercalation process. In addition to increasing the interlayer spacing (2.50 Å to 8.01 Å), the introduction of the DMSO molecule modifies the charge distribution in the kaolinite structure, which contributes to the increase in the amount of etheramine adsorbed. The protonated etheramine molecule interacts more effectively with the KDMSO.
中文翻译:

二甲亚砜插层高岭石对醚胺吸附的影响:实验和理论研究
这项研究评估了天然高岭石(KN)中二甲亚砜(DMSO)插层对醚胺吸附的影响。使用中心复合设计研究了参数pH,初始醚胺浓度和吸附剂质量的影响。使用响应面方法确定最佳条件。进行理论计算以优化高岭石,DMSO,KDMSO的几何形状,分子和质子化的醚胺结构,以及醚胺和高岭石之间的相互作用。XRD和FTIR结果证实了DMSO嵌入高岭石中,该嵌入过程引起等电点的显着增加。结果表明,对于两种吸附剂,在pH 10和400 mg L-1的醚胺浓度下,吸附效率均更高,而在0.1时,吸附效率更高。 克KN和0.2 克KDMSO。动力学数据最准确地拟合伪二阶模型。实验数据与等温线模型的拟合表明,Sips是最合适的模型。Eads的计算表明,KN和KDMSO去除醚胺的机理涉及化学吸附。可重复使用性测试确定,经过四次使用,醚胺的去除效率没有明显变化,因此可以将高岭石用于废水处理。理论研究使人们对插层过程有了更好的理解。除了增加层间间距( 2.50Å至8.01 ),引入DMSO分子会改变高岭石结构中的电荷分布,这有助于增加所吸收的醚胺数量。质子化的醚胺分子与KDMSO更有效地相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号