Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2021-04-14 , DOI: 10.1016/j.jece.2021.105498 Petr Praus , Aneta Smýkalová , Kryštof Foniok , Vlastimil Novák , Jan Hrbáč
|
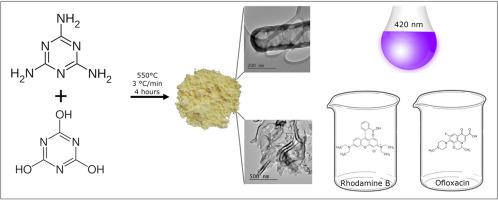
Bare and oxygen doped graphitic carbon nitride were synthetized by the thermal polymerization of melamine (CN-M) and the mechanical mixtures of melamine and cyanuric acid, respectively, at 550°C for 4 h. The ratios of melamine and cyanuric acid were 1:0.5, 1:1 and 1:2 (CN-MCA1, CN-MCA2 and CN-MCA3). The content of oxygen increased from 1.88 wt.% (CN-M) to 3.93 wt.% (CN-MCA3) and the specific surface area increased from 14 to 41 m2 g-1. The prepared CN materials were characterized by the elemental analysis, UV-Vis diffuse reflectance, X-ray diffraction, Fourier transform infrared spectrometry, the physisorption of nitrogen, scanning electron and high-resolution transmission electron microscopy, X-ray photoelectron spectroscopy, the determination of zeta potentials, the Mott-Schottky method
The addition of cyanuric acid led to partial changes in the CN morphology, as documented by occurrence of CN tubes, increase in the specific surface area and was a source of additional -O- and -OH moieties which modified the CN surface in addition to the spontaneous oxygenation observed in CN-M. The above effects positively influenced photocatalytic activity of the CN materials as demonstrated using Rhodamine B (RhB) and the Ofloxacin antibiotics. The photocatalytic decomposition of Ofloxacin was more efficient than that of RhB and differed in kinetics (first-order vs. zero-order reaction). The reusability and stability of the CN materials was verified by repeating batch photocatalytic decompositions of Ofloxacin experiments for five times.
中文翻译:

通过氰尿酸用氧掺杂石墨氮化碳:性质和光催化应用
分别通过三聚氰胺(CN-M)的热聚合以及三聚氰胺和氰尿酸的机械混合物在550°C下合成4 小时,来合成裸露的和掺杂氧的石墨氮化碳。三聚氰胺和氰尿酸的比例为1:0.5、1:1和1:2(CN-MCA1,CN-MCA2和CN-MCA3)。氧含量从1.88 wt。%(CN-M)增加到3.93 wt。%(CN-MCA3),比表面积从14增加到41 m 2 g -1。通过元素分析,UV-Vis漫反射,X射线衍射,傅立叶变换红外光谱,氮的物理吸附,扫描电子和高分辨率透射电子显微镜,X射线光电子能谱,测定来表征所制备的CN材料。电位,莫特-肖特基方法
氰尿酸的添加导致CN形态的部分变化,如CN管的出现所证明的,比表面积的增加,并且是除-O-和-OH部分外还修饰CN表面的来源。在CN-M中观察到自发氧合。如使用罗丹明B(RhB)和氧氟沙星抗生素所证明的,上述效果对CN材料的光催化活性产生了积极影响。氧氟沙星的光催化分解比RhB更为有效,并且动力学(一级反应与零级反应)不同。通过重复分批进行氧氟沙星实验的光催化分解五次,验证了CN材料的可重用性和稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号