当前位置:
X-MOL 学术
›
J. Synchrotron Radiat.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In situ X‐ray absorption spectroscopic studies of photocatalytic oxidation of As(III) to less toxic As(V) by TiO2 nanotubes
Journal of Synchrotron Radiation ( IF 2.4 ) Pub Date : 2021-04-13 , DOI: 10.1107/s1600577521003076 T.-L. Hsiung , L.-W. Wei , H.-L. Huang , Y.-J. Tuan , H. Paul Wang
Journal of Synchrotron Radiation ( IF 2.4 ) Pub Date : 2021-04-13 , DOI: 10.1107/s1600577521003076 T.-L. Hsiung , L.-W. Wei , H.-L. Huang , Y.-J. Tuan , H. Paul Wang
|
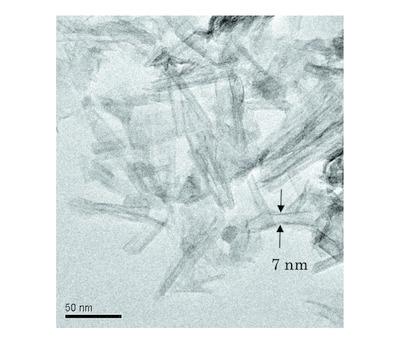
Arsenic in groundwater caused the black‐foot disease (BFD) in many countries in the 1950–1960s. It is of great importance to develop a feasible method for removal of arsenic from contaminated groundwater in BFD endemic areas. Photocatalytic oxidation of As(III) to less toxic As(V) is, therefore, of significance for preventing any arsenic‐related disease that may occur. By in situ synchrotron X‐ray absorption spectroscopy, the formation of As(V) is related to the expense of As(III) disappearance during photocatalysis by TiO2 nanotubes (TNTs). Under UV/Vis light irradiation, the apparent first‐order rate constant for the photocatalytic oxidation of As(III) to As(V) is 0.0148 min−1. It seems that As(III) can be oxidized with photo‐excited holes while the not‐recombined electrons may be scavenged with O2 in the channels of the well defined TNTs (an opening of 7 nm in diameter). In the absence of O2, on the contrary, As(III) can be reduced to As(0), to some extent. Cu(II) (CuO), as an electron acceptor, was impregnated on the TNTs surfaces in order to gain a better understanding of electron transfer during photocatalysis. It appears that As(III) can be oxidized to As(V) while Cu(II) is reduced to Cu(I) and Cu(0). The molecular‐scale data are very useful in revealing the oxidation states and interconversions of arsenic during the photocatalytic reactions. This work has implications in that the toxicity of arsenic in contaminated groundwater or wastewater can be effectively decreased via solar‐driven photocatalysis, which may facilitate further treatments by coagulation.
中文翻译:

TiO2 纳米管光催化氧化 As(III) 为毒性较小的 As(V) 的原位 X 射线吸收光谱研究
地下水中的砷在 1950-1960 年代在许多国家引起了黑足病 (BFD)。开发一种可行的方法去除 BFD 流行区受污染地下水中的砷具有重要意义。因此,将 As(III) 光催化氧化为毒性较小的 As(V) 对于预防可能发生的任何砷相关疾病具有重要意义。通过原位同步加速器 X 射线吸收光谱,As(V) 的形成与 TiO 2纳米管 (TNTs)光催化过程中 As(III) 消失的费用有关。在紫外/可见光照射下,As(III) 光催化氧化为 As(V) 的表观一级速率常数为 0.0148 min -1. 似乎 As(III) 可以被光激发空穴氧化,而未复合的电子可以在明确定义的 TNT(直径为 7 nm 的开口)的通道中被 O 2清除。在没有 O 2的情况下,相反,在某种程度上,As(III) 可以简化为 As(0)。Cu(II) (CuO) 作为电子受体,被浸渍在 TNTs 表面,以便更好地了解光催化过程中的电子转移。似乎 As(III) 可以被氧化为 As(V),而 Cu(II) 被还原为 Cu(I) 和 Cu(0)。分子尺度数据对于揭示光催化反应过程中砷的氧化态和相互转化非常有用。这项工作的意义在于,通过太阳能驱动的光催化可以有效降低受污染地下水或废水中砷的毒性,这可能有助于通过混凝进行进一步处理。
更新日期:2021-05-06
中文翻译:

TiO2 纳米管光催化氧化 As(III) 为毒性较小的 As(V) 的原位 X 射线吸收光谱研究
地下水中的砷在 1950-1960 年代在许多国家引起了黑足病 (BFD)。开发一种可行的方法去除 BFD 流行区受污染地下水中的砷具有重要意义。因此,将 As(III) 光催化氧化为毒性较小的 As(V) 对于预防可能发生的任何砷相关疾病具有重要意义。通过原位同步加速器 X 射线吸收光谱,As(V) 的形成与 TiO 2纳米管 (TNTs)光催化过程中 As(III) 消失的费用有关。在紫外/可见光照射下,As(III) 光催化氧化为 As(V) 的表观一级速率常数为 0.0148 min -1. 似乎 As(III) 可以被光激发空穴氧化,而未复合的电子可以在明确定义的 TNT(直径为 7 nm 的开口)的通道中被 O 2清除。在没有 O 2的情况下,相反,在某种程度上,As(III) 可以简化为 As(0)。Cu(II) (CuO) 作为电子受体,被浸渍在 TNTs 表面,以便更好地了解光催化过程中的电子转移。似乎 As(III) 可以被氧化为 As(V),而 Cu(II) 被还原为 Cu(I) 和 Cu(0)。分子尺度数据对于揭示光催化反应过程中砷的氧化态和相互转化非常有用。这项工作的意义在于,通过太阳能驱动的光催化可以有效降低受污染地下水或废水中砷的毒性,这可能有助于通过混凝进行进一步处理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号