Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2021-04-13 , DOI: 10.1016/j.jcou.2021.101532 Preeyaporn Poldorn , Yutthana Wongnongwa , Tanabat Mudchimo , Siriporn Jungsuttiwong
|
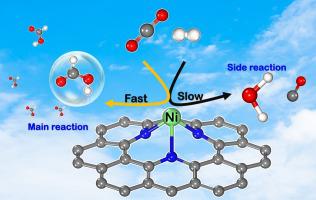
Developing highly efficient and cheap catalysts for the CO2 hydrogenation is the key to achieve CO2 conversion into clean energy. Herein, periodic density functional theory (DFT) calculations are performed to investigate possible reaction mechanisms for the hydrogenation of CO2 to formic acid (cis- or trans-HCOOH) product over a single Fe or Ni atom incorporated nitrogen-doped graphene (Fe-N3Gr or Ni-N3Gr) sheets. Our calculations found that the CO2 hydrogenation proceeds via a coadsorption mechanism to produce cis- or trans-HCOOH over Fe-N3Gr and Ni-N3Gr surfaces, which is classified into 2 steps: (1) the CO2 hydrogenation to form a formate (HCOO*) intermediate and (2) hydrogen abstraction to produce cis- or trans-HCOOH. The formation of trans-HCOOH over both Fe-N3Gr and Ni-N3Gr surfaces exhibit the obvious superiority due to the low barrier all through the whole channel. The highest energy barriers (Ea) in the case of trans-HCOOH formation on Fe-N3Gr and Ni-N3Gr surfaces are only 0.57 and 0.37 eV, respectively, which indicated that the CO2 hydrogenation to trans-HCOOH could be realized over these catalysts at low temperatures, especially the Ni-N3Gr surface. On the other hand, our findings show that the competitive reaction that produces CO and H2O is almost impossible or extremely difficult to proceeds under ambient conditions due to the large Ea for the formation of these side products. Moreover, the microkinetic modeling of the CO2 hydrogenation on both surfaces was investigated to confirm these results. Thus, the Fe-N3Gr and Ni-N3Gr catalysts reveal excellent catalytic activity and highly selective for CO2 hydrogenation to trans-HCOOH. This theoretical investigation not only provides a promising catalyst but also gives a deeper understanding of CO2 hydrogenation reaction.
中文翻译:

掺氮石墨烯在单原子(Fe或Ni)上催化CO 2加氢的理论见解
开发用于CO 2加氢的高效,廉价的催化剂是实现CO 2转化为清洁能源的关键。在此,进行周期性密度泛函理论(DFT)计算以研究在单个掺有氮或掺杂氮的石墨烯(Fe-)上将CO 2加氢成甲酸(顺式或反式-HCOOH)的可能反应机理。N3Gr或Ni-N3Gr)薄板。我们的计算发现,CO 2的氢化反应是通过共吸附机制在Fe-N3Gr和Ni-N3Gr表面上产生顺式或反式HCOOH的过程,该过程分为2个步骤:(1)CO 2氢化形成甲酸酯(HCOO *)中间体,(2)夺氢生成顺式或反式HCOOH。在Fe-N3Gr和Ni-N3Gr表面上形成反式HCOOH表现出明显的优越性,这是由于贯穿整个通道的低势垒所致。在Fe-N3Gr和Ni-N3Gr表面上形成反式HCOOH时,最高能垒(E a)分别仅为0.57和0.37 eV,这表明在这些条件下可以实现CO 2加氢成反式HCOOH催化剂在低温下,尤其是Ni-N3Gr表面。另一方面,我们的发现表明产生CO和H的竞争反应由于形成这些副产物的E a大,因此在环境条件下几乎不可能或很难进行2 O的反应。此外,研究了两个表面上CO 2加氢的微观动力学模型,以证实这些结果。因此,Fe-N3Gr和Ni-N3Gr催化剂显示出优异的催化活性,并且对CO 2加氢成反式HCOOH具有高度选择性。这一理论研究不仅提供了有希望的催化剂,而且使人们对CO 2加氢反应有了更深入的了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号