Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2021-04-12 , DOI: 10.1016/j.jece.2021.105478 Eli Syafiqah Aziman , Aznan Fazli Ismail
|
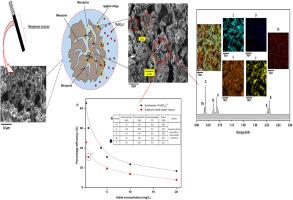
In this present study, thorium ion [Th(SO4)32-] was removed from aqueous sulphate medium using fabricated activated carbon-based electrodes (CBE). The thermal cross-linking technique was used to synthesize the CBE. Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) technique, X-ray photoelectron spectroscopy (XPS) and field emission scanning electron microscopy (FESEM) were used to characterize the CBE adsorbent. The adsorptive characteristics were investigated through batch static adsorption experiments by varying the concentration, time and applied voltage. The adsorption statistics were justified by isotherm and kinetic models. The Langmuir and Freundlich adsorption model was revealed to be fitted, with a maximum adsorption capacity of 8.4 mg-Th/ g-Carbon. The electrosorption of [Th(SO4)32-] onto the CBE followed the pseudo-first and second-order kinetic rate models. Through FESEM, XRD and XPS characterization studies, a suitable mechanism for thorium sorption has been postulated.
中文翻译:

通过电吸附快速选择性去除Thor,以有效管理稀土萃取残留物
在本研究中,or离子[Th(SO 4)3 2-使用制造的基于活性炭的电极(CBE)从硫酸盐介质中除去]。使用热交联技术来合成CBE。使用傅里叶变换红外光谱(FTIR),X射线衍射(XRD)技术,X射线光电子能谱(XPS)和场发射扫描电子显微镜(FESEM)表征了CBE吸附剂。通过改变浓度,时间和施加电压,通过间歇静态吸附实验研究了吸附特性。通过等温线和动力学模型证明了吸附统计数据是正确的。揭示了Langmuir和Freundlich吸附模型的拟合,最大吸附容量为8.4 mg-Th / g-碳。[Th(SO 4)3 2-的电吸附在CBE上跟随伪一阶和二阶动力学速率模型。通过FESEM,XRD和XPS表征研究,已经提出了a吸附的合适机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号