Journal of the Taiwan Institute of Chemical Engineers ( IF 5.5 ) Pub Date : 2021-04-09 , DOI: 10.1016/j.jtice.2021.03.050 Zichen Wang , Hongbin Li , Wei Ma , Yinglong Wang , Peizhe Cui , Jianguang Qi , Zhengrun Chen , Zhaoyou Zhu , Fanqing Meng
|
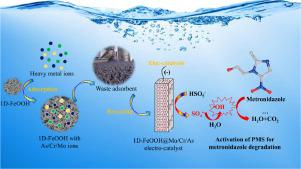
Peroxymonosulfate (PMS) activation have provided a new direction to eliminate the organic pollutant. Current challenges that must be solved include secondary pollution by the metal ions and limitation in the recyclability of the catalysts. The waste 1D-FeOOH adsorbent with adsorption of metal elements (As, Cr or Mo ions) has been reused as an efficient electro-catalyst for PMS activation and used to metronidazole (MTD) degradation for the first time. The effects of current density, initial reaction pH, stirring rate, PMS dosage and solution temperature on MTD degradation was studied. The used 1D-FeOOH with adsorption of Mo ions showed the highest PMS activation ability and 99.6% MTD could be removed after 30 min degradation. The •OH and Found that SO4•− is the main free radical for MTD degradation. DFT calculation and characterization results were demonstrated that the high PMS electro-catalysis activation ability of the 1D-FeOOH@Mo has resulted from the special composition-dependent interactions among FeOOH@Mo and PMS, including stable adsorption properties, easy electron transfer and weak O O bond. The possible PMS electro-catalysis activation mechanism and MTD degradation pathway have been proposed. (ECOSAR) was used to evaluate the toxicity of metronidazole during degradation. The results showed that the toxicity of MTD and its intermediates could be effectively reduced by prolonging the degradation time in 1D-FeOOH@Mo solution. This work provides new insights for waste adsorbent disposal and gives a new perspective for PMS activation to degradation of antibiotics in water.
O bond. The possible PMS electro-catalysis activation mechanism and MTD degradation pathway have been proposed. (ECOSAR) was used to evaluate the toxicity of metronidazole during degradation. The results showed that the toxicity of MTD and its intermediates could be effectively reduced by prolonging the degradation time in 1D-FeOOH@Mo solution. This work provides new insights for waste adsorbent disposal and gives a new perspective for PMS activation to degradation of antibiotics in water.
中文翻译:

“使用过的” As / Cr / Mo @ FeOOH材料对甲硝唑的高效电催化活化过硫酸单硫酸盐:降解机理和毒性评估
过氧一硫酸盐(PMS)活化为消除有机污染物提供了新的方向。当前必须解决的挑战包括金属离子的二次污染和催化剂可再循环性的限制。带有金属元素(As,Cr或Mo离子)吸附的1D-FeOOH废吸附剂已被重新用作PMS活化的有效电催化剂,并首次用于甲硝唑(MTD)降解。研究了电流密度,初始反应pH,搅拌速率,PMS用量和溶液温度对MTD降解的影响。所使用的具有吸附Mo离子的1D-FeOOH表现出最高的PMS活化能力,降解30分钟后可去除99.6%的MTD。•OH,发现SO 4 •-是MTD降解的主要自由基。DFT计算和表征结果表明,一维FeOOH @ Mo具有较高的PMS电催化活化能力,这是由于FeOOH @ Mo与PMS之间存在特殊的成分依赖性相互作用,包括稳定的吸附性能,易电子转移和弱O O键。提出了可能的PMS电催化活化机理和MTD降解途径。(ECOSAR)用于评估甲硝唑降解过程中的毒性。结果表明,延长1D-FeOOH @ Mo溶液的降解时间可以有效降低MTD及其中间体的毒性。这项工作为废物吸附剂的处理提供了新的见解,并为PMS活化降解水中的抗生素提供了新的视角。
O键。提出了可能的PMS电催化活化机理和MTD降解途径。(ECOSAR)用于评估甲硝唑降解过程中的毒性。结果表明,延长1D-FeOOH @ Mo溶液的降解时间可以有效降低MTD及其中间体的毒性。这项工作为废物吸附剂的处理提供了新的见解,并为PMS活化降解水中的抗生素提供了新的视角。











































 京公网安备 11010802027423号
京公网安备 11010802027423号