Autonomic Neuroscience ( IF 3.2 ) Pub Date : 2021-04-10 , DOI: 10.1016/j.autneu.2021.102807 Louiza Belkacemi 1 , Weixia Zhong 1 , Nissar A Darmani 1
|
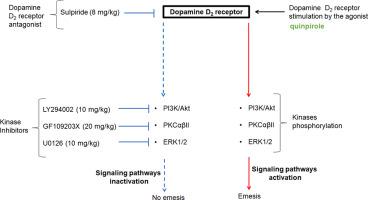
With its five receptor subtypes (D1–5), dopamine is implicated in a myriad of neurological illnesses. Dopamine D2 receptor-based agonist therapy evokes nausea and vomiting. The signaling mechanisms by which dopamine D2 receptors evoke vomiting remains unknown. Phosphatidylinositol 3-kinases (PI3K)- and protein kinase C (PKC)-related signaling cascades stimulate vomiting post-injection of various emetogens in emetically competent animals. This study investigated potential mechanisms involved in dopamine D2 receptor-mediated vomiting using least shrews. We found that vomiting evoked by the selective dopamine D2 receptor agonist quinpirole (2 mg/kg, i.p.) was significantly suppressed by: i) a dopamine D2 preferring antagonist, sulpiride (s.c.); ii) a selective PI3K inhibitor, LY294002 (i.p.); iii) a PKCαβII inhibitor, GF109203X (i.p.); and iv) a selective inhibitor of extracellular signal-regulated protein kinase1/2 (ERK1/2), U0126 (i.p.). Quinpirole-evoked c-fos immunofluorescence in the nucleus tractus solitarius (NTS) was suppressed by pretreatment with sulpiride (8 mg/kg, s.c.). Western blot analysis of shrew brainstem emetic loci protein lysates revealed a significant and time-dependent increase in phosphorylation of Akt (protein kinase B (PKB)) at Ser473 following a 30-min exposure to quinpirole (2 mg/kg, i.p.). Pretreatment with effective antiemetic doses of sulpiride, LY294002, GF109203X, or U0126 significantly reduced quinpirole-stimulated phosphorylation of emesis-associated proteins including p-85PI3K, mTOR (Ser2448/2481), PKCαβII (Thr638/641), ERK1/2 (Thr202/204), and Akt (Ser473). Our results substantiate the implication of PI3K/mTOR/Akt and PI3K/PKCαβII/ERK1/2/Akt signaling pathways in dopamine D2 receptor-mediated vomiting. Potential novel antiemetics targeting emetic proteins associated with these signaling cascades may offer enhanced potency and/or efficacy against emesis.
中文翻译:

最少鼩鼱 (Cryptotis parva) 中涉及多巴胺 D2 受体诱发呕吐的信号转导途径
凭借其五种受体亚型 (D 1 – 5 ),多巴胺与无数神经系统疾病有关。基于多巴胺 D 2受体的激动剂疗法会引起恶心和呕吐。多巴胺D 2受体引起呕吐的信号传导机制仍然未知。磷脂酰肌醇 3-激酶 (PI3K) 和蛋白激酶 C (PKC) 相关的信号级联反应在有呕吐能力的动物中刺激注射各种催吐剂后的呕吐。本研究使用最少的鼩鼱研究了涉及多巴胺 D 2受体介导的呕吐的潜在机制。我们发现选择性多巴胺 D 2诱发呕吐受体激动剂喹吡罗 (2 mg/kg, ip) 被以下因素显着抑制:i) 多巴胺 D 2首选拮抗剂舒必利(sc);ii) 选择性 PI3K 抑制剂 LY294002 (ip);iii) PKCαβII 抑制剂,GF109203X (ip);iv) 细胞外信号调节蛋白激酶 1/2 (ERK1/2)、U0126 (ip) 的选择性抑制剂。用舒必利 (8 mg/kg, sc) 预处理可抑制孤束核 (NTS) 中喹吡罗诱发的 c-fos 免疫荧光。鼩鼱脑干催吐基因座蛋白裂解物的蛋白质印迹分析显示,在与喹吡罗 (2 mg/kg, ip) 接触 30 分钟后,Akt(蛋白激酶 B (PKB))在 Ser473 位点的磷酸化显着且呈时间依赖性增加。用有效止吐剂量的舒必利、LY294002、GF109203X 或 U0126 预处理显着降低喹吡罗刺激的呕吐相关蛋白磷酸化,包括 p-85PI3K、mTOR (Ser2448/2481)、PKCαβII (Thr638/641)、ERK1/2 (Thr202/204) 和 Akt (Ser473)。我们的结果证实了 PI3K/mTOR/Akt 和 PI3K/PKCαβII/ERK1/2/Akt 信号通路在多巴胺 D 中的意义2受体介导的呕吐。针对与这些信号级联相关的催吐蛋白的潜在新型止吐剂可能会提供增强的抗呕吐效力和/或功效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号