当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Standard Thermochemical Characteristics of Combustion and Formation of Bulky Benzoquinone-Type Derivatives at T = 298.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-04-08 , DOI: 10.1021/acs.jced.0c01042 Kira I. Pashanova 1 , Polina E. Goryunova 2 , Semen S. Sologubov 2 , Alexey V. Markin 2 , Natalia N. Smirnova 2 , Alexandr V. Piskunov 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-04-08 , DOI: 10.1021/acs.jced.0c01042 Kira I. Pashanova 1 , Polina E. Goryunova 2 , Semen S. Sologubov 2 , Alexey V. Markin 2 , Natalia N. Smirnova 2 , Alexandr V. Piskunov 1
Affiliation
|
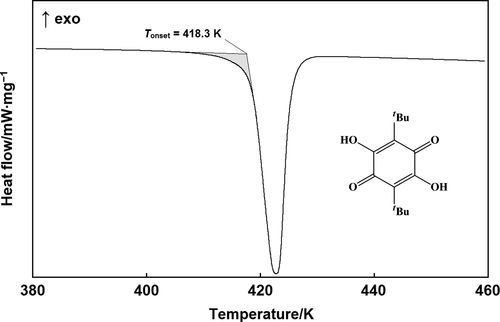
In the present work, the combustion energies of 2,5-dihydroxy-3,6-di-tert-butyl-p-benzoquinone and 4-methoxy-3,6-di-tert-butyl-o-benzoquinone were determined by static-bomb combustion calorimetry in oxygen at a reference temperature of 298.15 K. The standard molar enthalpies of combustion and formation of the studied benzoquinones were calculated using the experimental values of the combustion energies. The enthalpies of formation of the investigated compounds were compared with the thermochemical characteristics of the previously studied benzoquinone derivatives.
中文翻译:

在T = 298.15 K时燃烧和形成大体积苯醌型衍生物的标准热化学特征
在本工作中,燃烧能量2,5-二羟基-3,6-二-叔丁基- p醌和4-甲氧基-3,6-二-叔丁基- ö醌被静态确定的氧在参考温度298.15 K下的炸弹燃烧量热法。使用燃烧能的实验值,计算了标准的燃烧摩尔焓和所研究的苯醌的形成。将所研究化合物的形成焓与先前研究的苯醌衍生物的热化学特性进行了比较。
更新日期:2021-05-13
中文翻译:

在T = 298.15 K时燃烧和形成大体积苯醌型衍生物的标准热化学特征
在本工作中,燃烧能量2,5-二羟基-3,6-二-叔丁基- p醌和4-甲氧基-3,6-二-叔丁基- ö醌被静态确定的氧在参考温度298.15 K下的炸弹燃烧量热法。使用燃烧能的实验值,计算了标准的燃烧摩尔焓和所研究的苯醌的形成。将所研究化合物的形成焓与先前研究的苯醌衍生物的热化学特性进行了比较。










































 京公网安备 11010802027423号
京公网安备 11010802027423号