Journal of Structural Biology ( IF 3.0 ) Pub Date : 2021-04-08 , DOI: 10.1016/j.jsb.2021.107738 Steven Hayward 1 , E James Milner-White 2
|
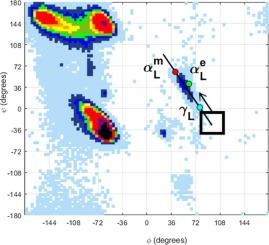
In amyloid diseases an insoluble amyloid fibril forms via a soluble oligomeric intermediate. It is this intermediate that mediates toxicity and it has been suggested, somewhat controversially, that it has the α-sheet structure. Nests and α-strands are similar peptide motifs in that alternate residues lie in the αR and γL regions of the Ramachandran plot for nests, or αR and αL regions for α-strands. In nests a concavity is formed by the main chain NH atoms whereas in α-strands the main chain is almost straight. Using “Ramachandran propensity plots” to focus on the αL/γL region, it is shown that glycine favours γL (82% of amino acids are glycine), but disfavours αL (3% are glycine). Most charged and polar amino acids favour αL with asparagine having by far the highest propensity. Thus, glycine favours nests but, contrary to common expectation, should not favour α-sheet. By contrast most charged or polar amino acids should favour α-sheet by their propensity for the αL conformation, which is more discriminating amongst amino acids than the αR conformation. Thus, these results suggest the composition of sequences that favour α-sheet formation and point towards effective prediction of α-sheet from sequence.
中文翻译:

使用 Ramachandran 倾向图确定有利于 αL 区域的氨基酸。α-折叠作为可能的淀粉样蛋白中间体的意义
在淀粉样蛋白疾病中,不溶性淀粉样蛋白原纤维通过可溶性寡聚中间体形成。正是这种中间体介导了毒性,有人提出,有点争议的是,它具有α-折叠结构。巢和 α 链是相似的肽基序,因为交替的残基位于拉马钱德兰图的 α R和 γ L区域中的巢,或 α R和 α L区域的 α 链。在嵌套中,主链 NH 原子形成凹面,而在 α 链中,主链几乎是直的。使用“Ramachandran 倾向图”关注 α L /γ L区域,表明甘氨酸有利于 γ L(82% 的氨基酸是甘氨酸),但不喜欢 α L(3% 是甘氨酸)。大多数带电和极性氨基酸偏爱α L,而天冬酰胺具有迄今为止最高的倾向。因此,甘氨酸有利于巢,但与普遍预期相反,不应该有利于 α-折叠。相比之下,大多数带电或极性氨基酸应该倾向于 α-折叠,因为它们倾向于 αL构象,这比αR构象更能区分氨基酸。因此,这些结果表明有利于α-折叠形成的序列组成,并指向从序列中有效预测α-折叠。











































 京公网安备 11010802027423号
京公网安备 11010802027423号