当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Arsenic Partitioning during Schwertmannite Dissolution and Recrystallization in the Presence of Fe(II) and Oxalic Acid
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-04-07 , DOI: 10.1021/acsearthspacechem.1c00009 Xiaohu Jin 1, 2 , Chuling Guo 1, 2 , Xiaofei Li 1, 2 , Yanguang Liao 1 , Qian Yao 1, 2 , Guining Lu 1, 2 , Zhi Dang 1, 2
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-04-07 , DOI: 10.1021/acsearthspacechem.1c00009 Xiaohu Jin 1, 2 , Chuling Guo 1, 2 , Xiaofei Li 1, 2 , Yanguang Liao 1 , Qian Yao 1, 2 , Guining Lu 1, 2 , Zhi Dang 1, 2
Affiliation
|
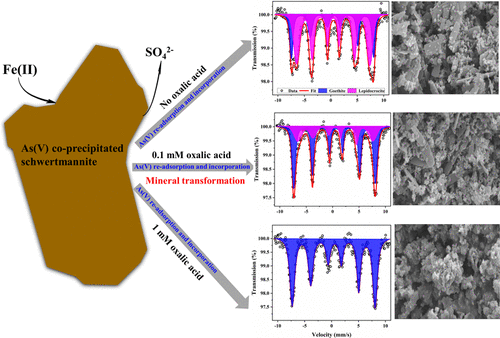
Fe(II)-driven transformation of schwertmannite can strongly impact the transport and redistribution of coprecipitated As in reducing acid sulfate soil (ASS)/acid mine drainage (AMD)-contaminated environments. Oxalic acid is prevalent in the natural environment, but its influence on Fe(II)-induced schwertmannite conversion and the repartitioning behavior of coprecipitated As is poorly characterized. In the present study, we examined the impact of oxalic acid on Fe(II)-induced As(V)-coprecipitated schwertmannite transformation and As redistribution between dissolved, adsorbed, and incorporated phases during mineral transformation at a pH of 5.5 and in 1 mM Fe(II). According to Mössbauer analysis, in 1 mM Fe(II) without oxalic acid treatment, schwertmannite was converted into lepidocrocite (65%) and goethite (35%). In mixed Fe(II)–oxalic acid systems, oxalic acid played a synergistic role in the dissolution and transformation of Fe(II)-induced schwertmannite. In 1 mM Fe(II) with 0.1/0.5/1 mM oxalic acid reaction systems after 14 day incubation, the fractions of goethite were 68% (lepidocrocite occupied 32%), 100%, and 100%, respectively. In all treatments, the PO43–-extractable fraction of As increased during mineral transformation, indicating that partially coprecipitated As of the original mineral transferred to the surface of the resulting products. Compared with mixed Fe(II)–oxalic acid systems, more adsorbed As formed in Fe(II) treatment without oxalic acid, which might correspond to the speciation of the resulting minerals. Although Fe(II) was a potential reductant for As(V), no As(III) was detected in any reaction system by the end of incubation. These findings have important implications for accurately predicting the geochemical behavior of As in AMD-contaminated areas.
中文翻译:

在Fe(II)和草酸存在下Schwertmannite溶解和重结晶过程中的砷分配
铁(II)驱动的schwertmannite的转化可在减少受酸性硫酸盐土壤(ASS)/酸性矿山排水(AMD)污染的环境中强烈影响共沉淀As的运输和再分布。草酸在自然环境中普遍存在,但其对Fe(II)诱导的Schwertmannite转化和共沉淀As的重分配行为的影响知之甚少。在本研究中,我们研究了草酸对Fe(II)诱导的As(V)共沉淀的Schwertmannite转化以及在pH为5.5和1 mM的矿物转化过程中溶解,吸附和结合相之间As的重新分布的影响铁(II)。根据Mössbauer分析,在未经草酸处理的1 mM Fe(II)中,schwertmannite转化为lepidocrocite(65%)和针铁矿(35%)。在Fe(II)-草酸混合体系中,草酸在Fe(II)诱导的Schwertmannite的溶解和转化中起着协同作用。孵育14天后,在具有0.1 / 0.5 / 1 mM草酸反应系统的1 mM Fe(II)中,针铁矿的比例分别为68%(铁云母占32%),100%和100%。在所有治疗中,PO4 3-可萃取的As分数在矿物转化过程中增加,表明原始矿物的部分共沉淀As转移到了最终产品的表面。与混合的Fe(II)-草酸体系相比,在没有草酸的Fe(II)处理中形成更多的吸附As,这可能对应于所形成矿物的形态。尽管Fe(II)是As(V)的潜在还原剂,但到培养结束时,在任何反应系统中均未检测到As(III)。这些发现对准确预测被AMD污染地区的砷的地球化学行为具有重要意义。
更新日期:2021-05-20
中文翻译:

在Fe(II)和草酸存在下Schwertmannite溶解和重结晶过程中的砷分配
铁(II)驱动的schwertmannite的转化可在减少受酸性硫酸盐土壤(ASS)/酸性矿山排水(AMD)污染的环境中强烈影响共沉淀As的运输和再分布。草酸在自然环境中普遍存在,但其对Fe(II)诱导的Schwertmannite转化和共沉淀As的重分配行为的影响知之甚少。在本研究中,我们研究了草酸对Fe(II)诱导的As(V)共沉淀的Schwertmannite转化以及在pH为5.5和1 mM的矿物转化过程中溶解,吸附和结合相之间As的重新分布的影响铁(II)。根据Mössbauer分析,在未经草酸处理的1 mM Fe(II)中,schwertmannite转化为lepidocrocite(65%)和针铁矿(35%)。在Fe(II)-草酸混合体系中,草酸在Fe(II)诱导的Schwertmannite的溶解和转化中起着协同作用。孵育14天后,在具有0.1 / 0.5 / 1 mM草酸反应系统的1 mM Fe(II)中,针铁矿的比例分别为68%(铁云母占32%),100%和100%。在所有治疗中,PO4 3-可萃取的As分数在矿物转化过程中增加,表明原始矿物的部分共沉淀As转移到了最终产品的表面。与混合的Fe(II)-草酸体系相比,在没有草酸的Fe(II)处理中形成更多的吸附As,这可能对应于所形成矿物的形态。尽管Fe(II)是As(V)的潜在还原剂,但到培养结束时,在任何反应系统中均未检测到As(III)。这些发现对准确预测被AMD污染地区的砷的地球化学行为具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号