当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selenium Isotope Shifts during the Oxidation of Selenide-Bearing Minerals
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-04-07 , DOI: 10.1021/acsearthspacechem.1c00036 Naomi L. Wasserman 1 , Kathrin Schilling 2 , Thomas M. Johnson 1 , Céline Pallud 3
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-04-07 , DOI: 10.1021/acsearthspacechem.1c00036 Naomi L. Wasserman 1 , Kathrin Schilling 2 , Thomas M. Johnson 1 , Céline Pallud 3
Affiliation
|
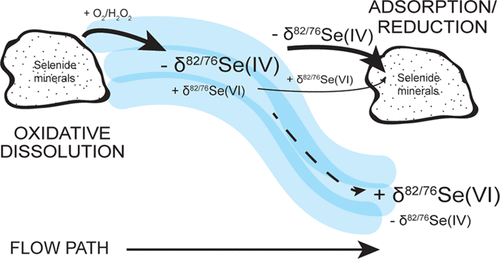
Oxidation of selenium (Se) largely drives the mobilization of soluble selenium oxyanions from rock and soil. Isotopic fractionation of selenium (82Se/76Se) has been used to track attenuating processes like reduction of Se(IV) and Se(VI). Isotopic shifts associated with oxidative dissolution of selenide-bearing minerals are poorly understood, despite their potential importance in determining 82Se/76Se of selenium sources and causing isotopic variation in contaminated systems. We examined 82Se/76Se of dissolved Se(IV) and Se(VI) during the oxidation of ferroselite (FeSe2) and berzelianite (Cu2Se) with low and high initial hydrogen peroxide (H2O2) concentrations in solution (0.007–1 mM) and under atmospheric oxygen levels. The 82Se/76Se of Se(VI) produced by oxidation in the experiments ranged from 1.5 to 14‰ greater than the initial minerals. These isotopic shifts arise from oxidation of Se(IV), reduction of Se(IV) or Se(VI) by mineral phases, and/or isotopic exchange between Se(IV) and Se(VI). At low concentrations of H2O2, isotopic fractionation associated with the reduction of Se(IV) to Se(0) on ferroselite is apparent. At high concentrations of H2O2, this process for ferroselite and berzelianite oxidation is either absent or diluted by a larger flux of Se(IV) created by rapid mineral oxidation. As Se(VI) is more mobile than Se(IV), our results suggest that oxidative weathering of selenium-bearing minerals, previously thought to induce minimal isotopic fractionation, tends to produce an isotopically heavy selenium weathering flux.
中文翻译:

硒化物矿物氧化过程中硒的同位素迁移
硒(Se)的氧化在很大程度上驱动了岩石和土壤中可溶性硒氧阴离子的迁移。硒的同位素分级分离(82 Se / 76 Se)已用于跟踪减毒过程,如还原Se(IV)和Se(VI)。尽管含硒矿物质氧化溶解相关的同位素迁移尽管了解其对确定硒源中82 Se / 76 Se的潜在重要性,并导致受污染系统中的同位素变化,却知之甚少。我们在氧化铁镁石(FeSe 2)和黄铁矿(Cu 2)的过程中检测了82 Se / 76 Se溶解的Se(IV)和Se(VI)(Se)在溶液中(0.007-1 -1 mM)以及在大气氧水平下具有较高和较低的初始过氧化氢(H 2 O 2)浓度。实验中通过氧化生成的Se(VI)的82 Se / 76 Se比初始矿物大1.5至14‰。这些同位素的转变是由于Se(IV)的氧化,矿物相对Se(IV)或Se(VI)的还原和/或Se(IV)和Se(VI)之间的同位素交换而引起的。在低浓度的H 2 O 2上,与硒铁上的Se(IV)还原为Se(0)有关的同位素分馏是显而易见的。在高浓度的H 2 O 2中,这种铁矿石和黄铁矿氧化的过程不存在,或者被快速矿物氧化产生的较大量的Se(IV)稀释。由于Se(VI)比Se(IV)更具流动性,因此我们的结果表明,以前认为引起同位素同位素分馏最少的含硒矿物的氧化风化趋向于产生同位素重的硒风化通量。
更新日期:2021-05-20
中文翻译:

硒化物矿物氧化过程中硒的同位素迁移
硒(Se)的氧化在很大程度上驱动了岩石和土壤中可溶性硒氧阴离子的迁移。硒的同位素分级分离(82 Se / 76 Se)已用于跟踪减毒过程,如还原Se(IV)和Se(VI)。尽管含硒矿物质氧化溶解相关的同位素迁移尽管了解其对确定硒源中82 Se / 76 Se的潜在重要性,并导致受污染系统中的同位素变化,却知之甚少。我们在氧化铁镁石(FeSe 2)和黄铁矿(Cu 2)的过程中检测了82 Se / 76 Se溶解的Se(IV)和Se(VI)(Se)在溶液中(0.007-1 -1 mM)以及在大气氧水平下具有较高和较低的初始过氧化氢(H 2 O 2)浓度。实验中通过氧化生成的Se(VI)的82 Se / 76 Se比初始矿物大1.5至14‰。这些同位素的转变是由于Se(IV)的氧化,矿物相对Se(IV)或Se(VI)的还原和/或Se(IV)和Se(VI)之间的同位素交换而引起的。在低浓度的H 2 O 2上,与硒铁上的Se(IV)还原为Se(0)有关的同位素分馏是显而易见的。在高浓度的H 2 O 2中,这种铁矿石和黄铁矿氧化的过程不存在,或者被快速矿物氧化产生的较大量的Se(IV)稀释。由于Se(VI)比Se(IV)更具流动性,因此我们的结果表明,以前认为引起同位素同位素分馏最少的含硒矿物的氧化风化趋向于产生同位素重的硒风化通量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号