当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct amidation of metallaaromatics: access to N-functionalized osmapentalynes via a 1,5-bromoamidated intermediate
Chemical Science ( IF 8.4 ) Pub Date : 2021-4-7 , DOI: 10.1039/d1sc01571k Hongjian Wang 1 , Yonghong Ruan 1 , Yu-Mei Lin 1 , Haiping Xia 1, 2
Chemical Science ( IF 8.4 ) Pub Date : 2021-4-7 , DOI: 10.1039/d1sc01571k Hongjian Wang 1 , Yonghong Ruan 1 , Yu-Mei Lin 1 , Haiping Xia 1, 2
Affiliation
|
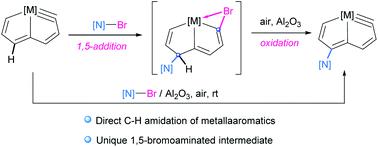
The direct C–H amidation or imidation of metallaaromatics with N-bromoamides or imides has been achieved under mild conditions and leads to the formation of a family of N-functionalized metallapentalyne derivatives. A unique 1,5-bromoamidated species has been identified, and can be viewed as a σH-adduct intermediate in a nucleophilic aromatic substitution. The 1,5-addition of both electrophilic and nucleophilic moieties into the metallaaromatic framework demonstrates a novel pathway in contrast to the typical radical process of arene C–H amidation involving N-haloamide reagents.
中文翻译:

金属芳烃的直接酰胺化:通过 1,5-溴酰胺化中间体获得 N-功能化的 osmapentalynes
金属芳烃与N-溴酰胺或酰亚胺的直接 C-H 酰胺化或酰亚胺化已在温和条件下实现,并导致形成N-官能化金属戊炔衍生物家族。已鉴定出一种独特的 1,5-溴酰胺化物质,可将其视为亲核芳族取代中的 σ H-加合物中间体。与涉及N-卤代酰胺试剂的芳烃 C-H 酰胺化的典型自由基过程相比,亲电和亲核部分的 1,5-加成到金属芳族骨架中证明了一种新的途径。
更新日期:2021-04-08
中文翻译:

金属芳烃的直接酰胺化:通过 1,5-溴酰胺化中间体获得 N-功能化的 osmapentalynes
金属芳烃与N-溴酰胺或酰亚胺的直接 C-H 酰胺化或酰亚胺化已在温和条件下实现,并导致形成N-官能化金属戊炔衍生物家族。已鉴定出一种独特的 1,5-溴酰胺化物质,可将其视为亲核芳族取代中的 σ H-加合物中间体。与涉及N-卤代酰胺试剂的芳烃 C-H 酰胺化的典型自由基过程相比,亲电和亲核部分的 1,5-加成到金属芳族骨架中证明了一种新的途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号