Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2021-04-06 , DOI: 10.1016/j.jece.2021.105433 Lijia Dong , Qing Liao , Chunlei Wu , Kui Du , Guodong Sheng
|
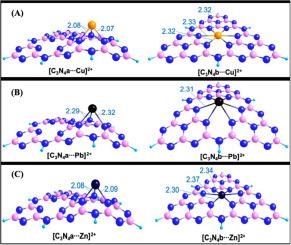
The graphitic phase carbon nitride (g-C3N4) is increasingly employed to remove heavy metals from wastewater, but the intrinsic imobilization mechanism still needs to be explored. Herein, the g-C3N4 before and after adsorption for Cu2+, Pb2+ and Zn2+ was firstly characterized using high resolution transmission electron microscope, energy dispersive X-ray, fourier transform infrared, and X-ray diffraction. Then two types of C3N4 structures were calculated, i.e. the triazine (C3N4a) and tri-s-triazine units (C3N4b) using the DFT theoretical calculation. The results indicated that the g-C3N4 materials possessed a well-developed 2D lamellar sturcture, basic tris-triazine units, and abundent C-N, C=N, –NH–, and –NH2 groups. After scuccessfully adsorption for Cu2+, Pb2+ and Zn2+, the surface functional groups, typical tris-triazine units, and the amino functions of g-C3N4 were still well retained. The Cu2+, Pb2+ and Zn2+ ions combined with C3N4a and C3N4b, trapped in the hole surrounded by trazine molecules, and produced both three-cordinated [C3N4a-Cu/Pb/Zn]2+ and six-coordination [C3N4b-Cu/Pb/Zn]2+ structures. Interestingly, the heavy metals could be trapped within the C3N4b plane but be fixed above the C3N4a plane, and a higher sorpiton energy and coordination number were found for the structure of [C3N4b-Cu/Pb/Zn]2+ compared to [C3N4a-Cu/Pb/Zn]2+. In total, this study provides a new insight into the adsorption mechanism of g-C3N4 for heavy metal ions in water.
中文翻译:

结合光谱表征和DFT理论计算,深入了解g 2 3吸附Cu 2 +,Pb 2+和Zn 2+到gC 3 N 4表面
越来越多地采用石墨相氮化碳(gC 3 N 4)去除废水中的重金属,但内在固定化机理仍需探索。在此,首先利用高分辨率透射电子显微镜,能量色散X射线,傅立叶变换红外和X射线衍射表征了Cu 2 +,Pb 2+和Zn 2+吸附前后的gC 3 N 4。然后计算出两种类型的C 3 N 4结构,即三嗪(C 3 N 4 a)和三-s-三嗪单元(C 3 N 4b)使用DFT理论计算。结果表明,gC 3 N 4材料具有发达的2D层状结构,基本的tris-triazine单元以及丰富的CN,C = N,-NH-和-NH 2基团。在成功吸附Cu 2 +,Pb 2+和Zn 2+之后,仍保留了gC 3 N 4的表面官能团,典型的tris-triazine单元和氨基官能团。Cu 2 +,Pb 2+和Zn 2+离子与C 3 N 4 a和C 3 N 4结合b,陷于被三嗪分子包围的孔中,并产生了三配位的[C 3 N 4 a-Cu / Pb / Zn] 2+和六配位的[C 3 N 4 b-Cu / Pb / Zn] 2 +结构。有趣的是,重金属可能被捕集在C 3 N 4 b平面内,但被固定在C 3 N 4 a平面上方,并且发现[C 3 N 4 b-Cu]结构具有更高的索顿能和配位数。/ Pb / Zn] 2+与[C 3 N 4 a-Cu / Pb / Zn] 2+。总的来说,这项研究为gC 3 N 4对水中重金属离子的吸附机理提供了新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号