Applied Geochemistry ( IF 3.1 ) Pub Date : 2021-04-06 , DOI: 10.1016/j.apgeochem.2021.104957 Dongli Li , Guoping Zhang , Qingyun Wang , Shirong Liu , Chao Ma , Jingjing Chen , Fengjuan Liu
|
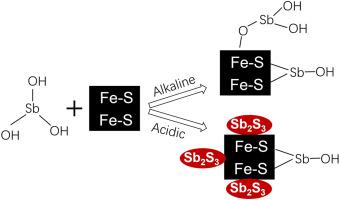
Ferrous sulfide (FeS) is an important carrier for metal(loid)s in anoxic environments, but the effect of FeS on the behavior of Sb is poorly understood. This work investigated the interaction of Sb(III) with FeS under anoxic condition. FeS was synthesized and both equilibrium and kinetic experiments on the interaction of Sb(III) with synthesized FeS were conducted at various pH values. The final solid phases were examined by SEM, XRD, TEM, and XPS. The results showed that the uptake of Sb(III) by FeS increased as pH decreased. The kinetic experiment at pH 5 obviously showed that the temporal decrease of Sb(III)aq coincide with the partial dissolution of FeS. In contrast, both the concentrations of Sb(III)aq and FeS in the kinetic experiment at pH 9 did not vary with time. The examination of the solid phases revealed the formation of amorphous Sb2S3 in the experiments at pH 5 and 7.5. Different mechanisms were suggested to affect the interaction of Sb(III) with FeS under acidic and alkaline conditions. At pH 9, adsorption dominated in the interaction. The decrease of Sb(III)aq and the concomitant partial dissolution of FeS at pH 5 indicated the replacement of FeS by Sb2S3, which was more significant at lower pH. The replacement of FeS by Sb2S3 was a relatively slow process compared to the acidic dissolution of FeS. The result of this study helps understand the mobility of Sb in anoxic environments and may favor the remediation of Sb contamination by the use of FeS.
中文翻译:

锑(III)水溶液与合成硫化亚铁的相互作用
硫化亚铁(FeS)是缺氧环境中金属(金属)的重要载体,但人们对FeS对Sb行为的影响知之甚少。这项工作研究了缺氧条件下Sb(III)与FeS的相互作用。合成了FeS,并在各种pH值下进行了Sb(III)与合成FeS相互作用的平衡和动力学实验。通过SEM,XRD,TEM和XPS检查最终的固相。结果表明,FeS对Sb(III)的吸收随着pH的降低而增加。pH为5的动力学实验明显表明,Sb(III)aq的时间减少与FeS的部分溶解相吻合。相反,两种浓度的Sb(III)水溶液pH值为9的动力学实验中,FeS和FeS不会随时间变化。固相的检查表明在pH 5和7.5的实验中形成了无定形的Sb 2 S 3。有人提出了在酸性和碱性条件下影响Sb(III)与FeS相互作用的不同机制。在pH 9时,吸附在相互作用中占主导。Sb(III)水溶液的减少以及在pH 5时FeS的部分溶解表明FeS被Sb 2 S 3取代,在较低pH值下更显着。用Sb 2 S 3代替FeS与FeS的酸性溶解相比,这是一个相对缓慢的过程。这项研究的结果有助于了解Sb在缺氧环境中的迁移性,并且可能有助于通过使用FeS来补救Sb污染。









































 京公网安备 11010802027423号
京公网安备 11010802027423号