Journal of Water Process Engineering ( IF 6.3 ) Pub Date : 2021-04-05 , DOI: 10.1016/j.jwpe.2021.102050 Muhammad Ali Inam , Rizwan Khan , Muhammad Waleed Inam , Ick Tae Yeom
|
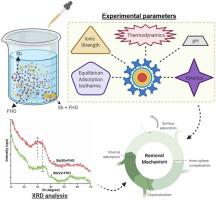
Antimony (Sb) is a regulated pollutant that is frequently present at elevated concentrations in natural bodies of water due to natural sources and human activities. Therefore, its purification from fresh water streams is of ultimate importance. This study focused on the iron hydroxide (FHO) formation and removal of Sb ions from water via ferric chloride (FC) coagulation. The effects of pH, contact time, concentration, temperature, and ionic strength (IS) were studied to explore the mechanistic insights into the removal behavior of both Sb ions during potable water purification. Results indicated that the solubility of FHO decrease by increasing the pH, thus repulsing the oxyanions at alkaline pH, decreasing in turn the adsorption capacity of Sb(V) species. The influence of contact time and equilibrium Sb concentration on FHO formation was found to be insignificant. The adsorption of Sb on FHO was well fitted with Pseudo-first order and Langmuir-Freundlich (L–F) models. Physical adsorption of Sb ions onto FHO was identified to be the major mechanism using the Dubinin-Radushkevich (D–R) isotherm model. Thermodynamic parameters (ΔG, ΔH, and ΔS) suggested that the Sb adsorption process was spontaneously exothermic with increased randomness. A more pronounced effect of IS on high Sb feed was observed with increasing sorption affinities upon enhancing electrolyte concentration. The mechanism was further supported by X-ray diffraction spectroscopy, which revealed the likelihood of inner-sphere complexation and physisorption of Sb onto FHO. Moreover, an involvement of coprecipitation phenomena during growing FHO was observed as key phenomena for greater Sb(III) removal. In general, the results of current research may provide detailed insights into the adsorption ability of FHO and the potential mechanisms responsible for the removal of Sb ions during potable water treatment.











































 京公网安备 11010802027423号
京公网安备 11010802027423号