Journal of Molecular Graphics and Modelling ( IF 2.7 ) Pub Date : 2021-04-05 , DOI: 10.1016/j.jmgm.2021.107913 M Shahamirian 1 , S M Azami 2
|
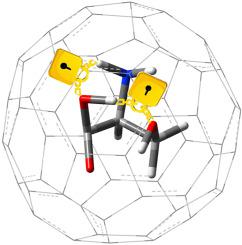
Intramolecular hydrogen bonding is evaluated in three different amino acids encapsulated in C60 fullerene in the context of electron density analysis. While conventional intramolecular hydrogen bonding in isolated amino acids are dominated by electrostatic character, it is shown that strong intramolecular hydrogen bonding can be formed in confined amino acids so that in two cases covalent intramolecular hydrogen bonding is appeared in the confined species. Also, results show that zwitterionic amino acids are stable in confined state, where no implicit or explicit solvation is applied. Covalent character for intramolecular hydrogen bonding in amino acids have not yet been reported.
中文翻译:

受限氨基酸中的强分子内氢键
在电子密度分析的背景下,在C 60富勒烯中封装的三种不同氨基酸中评估了分子内氢键。尽管分离的氨基酸中的常规分子内氢键主要由静电特性决定,但显示出可以在受限氨基酸中形成强分子内氢键,从而在两种情况下,在受限物种中出现共价分子内氢键。而且,结果表明,两性离子氨基酸在受限状态下是稳定的,其中未施加任何隐式或显式溶剂化。氨基酸中分子内氢键的共价特性尚未见报道。







































 京公网安备 11010802027423号
京公网安备 11010802027423号