Marine Chemistry ( IF 3.0 ) Pub Date : 2021-04-03 , DOI: 10.1016/j.marchem.2021.103965 Jonathan D. Sharp , Robert H. Byrne
|
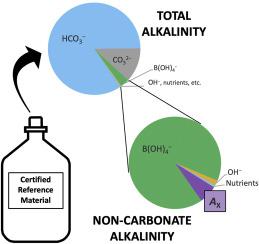
Certified reference materials (CRMs) for oceanic carbonate system measurements are critical for verifying the accuracy of laboratory protocols and the reliability of field sensors. CRMs are certified for total alkalinity and dissolved inorganic carbon, parameters that are (1) stable for a long period of time when a sample is properly stored and (2) not affected by changes in temperature and pressure. In experimentation initially designed to measure the total boron to salinity ratio of seawater, an interesting result has emerged regarding CRMs. A unique acidimetric titration method has indicated that three different batches of CRM contain excess alkalinity (i.e., alkalinity that is not attributable to inorganic bases included in the traditional definition of seawater total alkalinity) that is statistically greater than the excess alkalinity measured in open-ocean water from the Gulf of Mexico. Further, the amount of excess alkalinity appears to differ in certain CRM batches. Excess alkalinity in CRMs is likely caused by organic proton acceptors that are not completely oxidized by the ultraviolet sterilization procedure that CRMs undergo. The primary use of CRMs — to maintain the accuracy and consistency of carbonate system measurements — may be inhibited by excess alkalinity, which can cause differences in total alkalinity values determined by different titration methods. Excess alkalinity also invalidates the assumptions applied to marine CO2 system calculations, and so would produce incorrect values of CO2 system parameters calculated from certified total alkalinity and dissolved inorganic carbon values of CRMs. Finally, excess alkalinity analyses highlight the urgent need for the marine chemistry community to establish a universally agreed upon total boron to salinity ratio.
中文翻译:

技术说明:碳酸盐体系参考材料中的碱度过高
用于海洋碳酸盐系统测量的认证参考材料(CRM)对于验证实验室协议的准确性和现场传感器的可靠性至关重要。CRM通过了总碱度和溶解无机碳的认证,这些参数(1)正确保存样品后可长期稳定,(2)不受温度和压力变化的影响。在最初旨在测量海水中总硼盐度比的实验中,关于CRM的有趣结果出现了。独特的酸滴定法表明,三批不同的CRM含有过量的碱度(即,传统意义上的海水总碱度定义不归因于无机碱的碱度,其统计上大于来自墨西哥湾的开阔海洋水中测得的过量碱度。此外,在某些CRM批次中,过量碱度似乎有所不同。CRM中过多的碱度很可能是由于有机质子受体引起的,而该有机质子受体没有被CRM经历的紫外线灭菌程序完全氧化。CRM的主要用途-保持碳酸盐系统测量的准确性和一致性-可能会因碱度过高而受到抑制,碱度过高会导致由不同滴定方法确定的总碱度值出现差异。过多的碱度也使适用于海洋CO的假设无效 在某些CRM批次中,过量碱度的数量似乎有所不同。CRM中过多的碱度很可能是由于有机质子受体引起的,而该有机质子受体没有被CRM经历的紫外线灭菌程序完全氧化。CRM的主要用途-保持碳酸盐系统测量的准确性和一致性-可能会因碱度过高而受到抑制,碱度过高会导致由不同滴定方法确定的总碱度值出现差异。过多的碱度也使适用于海洋CO的假设无效 在某些CRM批次中,过量碱度的数量似乎有所不同。CRM中过多的碱度很可能是由于有机质子受体引起的,而该有机质子受体并未因CRM所经历的紫外线灭菌程序而被完全氧化。CRM的主要用途-保持碳酸盐系统测量的准确性和一致性-可能会因碱度过高而受到抑制,碱度过高会导致由不同滴定方法确定的总碱度值出现差异。过多的碱度也使适用于海洋CO的假设无效 CRM的主要用途-保持碳酸盐系统测量的准确性和一致性-可能会因碱度过高而受到抑制,碱度过高会导致由不同滴定方法确定的总碱度值出现差异。过多的碱度也使适用于海洋CO的假设无效 CRM的主要用途-保持碳酸盐系统测量的准确性和一致性-可能会因碱度过高而受到抑制,碱度过高会导致由不同滴定方法确定的总碱度值出现差异。过多的碱度也使适用于海洋CO的假设无效2系统计算,因此将产生错误的CO 2系统参数值,这些值是由CRM的总认证碱度和溶解无机碳值计算得出的。最后,过量的碱度分析突显了海洋化学界迫切需要建立普遍同意的总硼盐度比。










































 京公网安备 11010802027423号
京公网安备 11010802027423号