Journal of Water Process Engineering ( IF 6.3 ) Pub Date : 2021-04-02 , DOI: 10.1016/j.jwpe.2021.102052 Luciano Bernardo José , Ana Cláudia Queiroz Ladeira
|
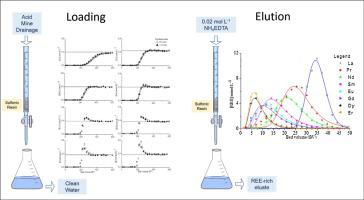
Considered critical and strategic, the rare earth elements (REE) can be found in ores as well as in secondary sources such as acid mine drainage (AMD). The present work studies the ion exchange process to recover and separate the REE present in a solution similar to an acid mine effluent containing La, Pr, Nd, Sm, Eu, Gd, Dy, Er, metal impurities (Al, Ca, Mg) and sulfate (SO42−). The loading experiments were carried out in columns filled with a strong acid cation resin. The feed solution contained 3.13 mmol L−1 of REE, 1.17 mmol L−1 of metal impurities and 11.6 mmol L−1 of sulfate, at pH 3.5. The elution/fractionation experiments were performed with NH4EDTA at 0.02, 0.05 and 0.30 mol L−1. The loading efficiencies were equal to 85 % (0.94 mmol g−1) for the REE and 30 % (0.15 mmol g−1) for the metal impurities. During loading, part of heavy REE and impurities were replaced by light REE. The best REE fractionation condition was obtained when 0.02mol L−1 NH4EDTA was employed. It was possible to separate the REE from Al and Ca. The final liquor contained 39.0 mmol L−1 of REE and 2.79 mmol L−1 of metal impurities. The process overall recovery was 83 %. The LEWATIT ® MDS 200H resin has shown to be suitable to recover and partially fractionate REE, including the separation of Sm, which has an ionic radius like Eu.
中文翻译:

使用强酸树脂从酸性矿山排水状溶液中回收和分离稀土元素
稀土元素(REE)被认为具有关键性和战略意义,可在矿石以及酸性矿山排水(AMD)等次要资源中找到。本工作研究离子交换过程,以回收和分离溶液中的稀土元素,该溶液类似于含有La,Pr,Nd,Sm,Eu,Gd,Dy,Er和金属杂质(Al,Ca,Mg)的酸性矿山废水。和硫酸盐(SO 4 2-)。加载实验是在装有强酸阳离子树脂的色谱柱中进行的。进料溶液在pH 3.5下含有3.13mmol L -1的REE,1.17mmol L -1的金属杂质和11.6mmol L -1的硫酸盐。用NH 4 EDTA在0.02、0.05和0.30 mol L下进行洗脱/分离实验-1。稀土的负载效率等于85%(0.94 mmol g -1),金属杂质的负载效率等于30%(0.15 mmol g -1)。在装载过程中,部分重质稀土元素和杂质被轻质稀土元素代替。当使用0.02mol L -1 NH 4 EDTA时,获得最佳的REE分离条件。可以将REE与Al和Ca分开。最终的液体包含39.0mmol L -1的REE和2.79mmol L -1的金属杂质。该方法的总回收率为83%。LEWATIT®MDS 200H树脂已被证明适用于回收和部分分离REE,包括Sm的分离,Sm的离子半径类似于Eu。











































 京公网安备 11010802027423号
京公网安备 11010802027423号