当前位置:
X-MOL 学术
›
Genes Cells
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
14–3-3γ prevents centrosome duplication by inhibiting NPM1 function
Genes to Cells ( IF 1.3 ) Pub Date : 2021-04-04 , DOI: 10.1111/gtc.12848 Arunabha Bose 1, 2 , Kruti Modi 1 , Suchismita Dey 3 , Somavally Dalvi 1 , Prafful Nadkarni 1 , Mukund Sudarshan 1, 2 , Tapas K Kundu 3 , Prasanna Venkatraman 1, 2 , Sorab N Dalal 1, 2
Genes to Cells ( IF 1.3 ) Pub Date : 2021-04-04 , DOI: 10.1111/gtc.12848 Arunabha Bose 1, 2 , Kruti Modi 1 , Suchismita Dey 3 , Somavally Dalvi 1 , Prafful Nadkarni 1 , Mukund Sudarshan 1, 2 , Tapas K Kundu 3 , Prasanna Venkatraman 1, 2 , Sorab N Dalal 1, 2
Affiliation
|
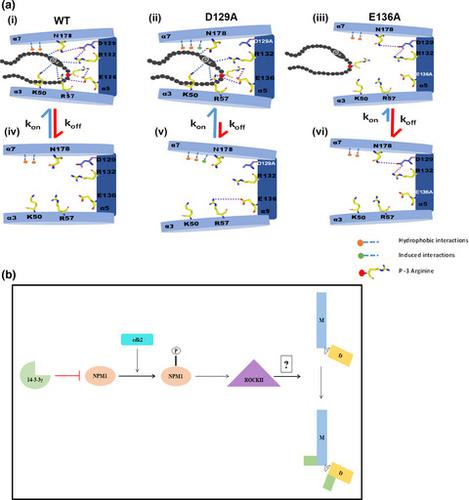
14–3–3 proteins bind to ligands via phospho-serine containing consensus motifs. However, the molecular mechanisms underlying complex formation and dissociation between 14–3–3 proteins and their ligands remain unclear. We identified two conserved acidic residues in the 14–3–3 peptide-binding pocket (D129 and E136) that potentially regulate complex formation and dissociation. Altering these residues to alanine led to opposing effects on centrosome duplication. D129A inhibited centrosome duplication, whereas E136A stimulated centrosome amplification. These results were due to the differing abilities of these mutant proteins to form a complex with NPM1. Inhibiting complex formation between NPM1 and 14–3-3γ led to an increase in centrosome duplication and over-rode the ability of D129A to inhibit centrosome duplication. We identify a novel role of 14–3-3γ in regulating centrosome licensing and a novel mechanism underlying the formation and dissociation of 14–3–3 ligand complexes dictated by conserved residues in the 14–3–3 family.
中文翻译:

14-3-3γ 通过抑制 NPM1 功能防止中心体复制
14-3-3 蛋白质通过包含共有基序的磷酸丝氨酸与配体结合。然而,14-3-3 蛋白质与其配体之间复合物形成和解离的分子机制仍不清楚。我们在 14-3-3 肽结合口袋(D129 和 E136)中鉴定了两个保守的酸性残基,它们可能调节复合物的形成和解离。将这些残基改变为丙氨酸会导致对中心体复制的相反影响。D129A 抑制中心体复制,而 E136A 刺激中心体扩增。这些结果是由于这些突变蛋白与 NPM1 形成复合物的能力不同。抑制 NPM1 和 14-3-3γ 之间的复合物形成导致中心体复制增加并过度抑制 D129A 抑制中心体复制的能力。
更新日期:2021-06-07
中文翻译:

14-3-3γ 通过抑制 NPM1 功能防止中心体复制
14-3-3 蛋白质通过包含共有基序的磷酸丝氨酸与配体结合。然而,14-3-3 蛋白质与其配体之间复合物形成和解离的分子机制仍不清楚。我们在 14-3-3 肽结合口袋(D129 和 E136)中鉴定了两个保守的酸性残基,它们可能调节复合物的形成和解离。将这些残基改变为丙氨酸会导致对中心体复制的相反影响。D129A 抑制中心体复制,而 E136A 刺激中心体扩增。这些结果是由于这些突变蛋白与 NPM1 形成复合物的能力不同。抑制 NPM1 和 14-3-3γ 之间的复合物形成导致中心体复制增加并过度抑制 D129A 抑制中心体复制的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号