Atmospheric Environment ( IF 4.2 ) Pub Date : 2021-04-03 , DOI: 10.1016/j.atmosenv.2021.118392 Naixian Wang , Jianfei Sun , Bo Wei , Qiong Mei , Zexiu An , Mingxue Li , Zhaoxu Qiu , Xiaofei Bo , Maoxia He
|
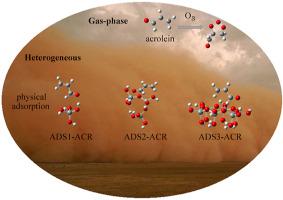
In this paper, the gas-phase and heterogeneous reactions of acrolein with ozone were studied comprehensively and deeply. Acrolein is one of the simplest unsaturated aldehydes found widely in the fuel combustion and industrial emissions. Mineral particles produced by frequent sandstorms have important effects on the tropospheric chemistry and the climate change. The content of silica in clay minerals is rich, thus three kinds of clusters (the monomeric model Si(OH)4, the linear model Si3O2(OH)8 and the cyclic hexamer model Si6O6(OH)12) were selected for the heterogeneous study. The reaction rate constant of acrolein with ozone at 298 K and 1 atm was 7.46 × 10−18 cm3 molecule−1 s−1, which was in agreement with the experimental value. SiO2 clusters interacted with acrolein via the hydrogen bonds and had good adsorption properties. The hydrogen bond between the oxygen of aldehyde group on acrolein and the hydrogen of silica surface was stronger and the adsorption energy was greater. The adsorption of silanol groups did not change the ozonation mechanism of acrolein, but had effects on the reaction rate. Among them, three silanol groups acted as positive catalysts. The kinetic data within the temperature range of 216.65–288.15 K corresponding to the height of the troposphere at 0–11 km showed that the rate constant of O3-initiated reaction of acrolein is positively correlated with the temperature. In addition, we also studied the reaction mechanism and kinetic of the Criegee intermediates reacting with atmospheric small molecules and isomerization process in gas-phase and heterogeneous processes.
中文翻译:

丙烯醛与臭氧的机理和动力学的气态和非均相反应
本文对丙烯醛与臭氧的气相反应和非均相反应进行了全面而深入的研究。丙烯醛是在燃料燃烧和工业排放中广泛发现的最简单的不饱和醛之一。频繁的沙尘暴产生的矿物颗粒对对流层化学和气候变化具有重要影响。粘土矿物中的二氧化硅含量丰富,因此存在三种簇(单体模型Si(OH)4,线性模型Si 3 O 2(OH)8和环状六聚体模型Si 6 O 6(OH)12)选择)进行异构研究。丙烯醛与臭氧在298 K和1个大气压下的反应速率常数为7.46×10 -18 cm 3分子-1 s -1,与实验值一致。二氧化硅2团簇通过氢键与丙烯醛相互作用,并具有良好的吸附性能。丙烯醛上的醛基的氧原子与二氧化硅表面的氢原子之间的氢键较强,吸附能较大。硅烷醇基团的吸附并没有改变丙烯醛的臭氧化机理,但对反应速率有影响。其中,三个硅烷醇基团充当正催化剂。在216.65–288.15 K温度范围内的动力学数据(对应于0-11 km的对流层高度)显示,O 3的速率常数丙烯醛引发的反应与温度呈正相关。此外,我们还研究了Criegee中间体与大气小分子反应的反应机理和动力学,以及气相和非均相过程中的异构化过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号