Atmospheric Environment ( IF 4.2 ) Pub Date : 2021-04-01 , DOI: 10.1016/j.atmosenv.2021.118383 Anna G. Zborowska , Ceara Y. MacInnis , Connie Z. Ye , Hans D. Osthoff
|
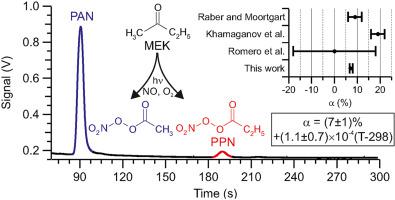
The methyl ethyl ketone (MEK) photolysis branching ratio (α) was re-evaluated by an end product analysis and box model simulations with the Master Chemical Mechanism (MCM). Using light emitting diodes centered at 285 nm or 315 nm, MEK was irradiated in the presence of nitric oxide and oxygen to produce peroxyacetic and peroxypropanoic nitric anhydride, CH3C(O)O2NO2 (PAN) and C2H5C(O)O2NO2 (PPN), which were quantified by gas chromatography. Box model simulations indicated that PPN is partially produced as a secondary product from chemistry initiated by reaction of the hydroxyl radical (OH) with MEK. Under NOx-limited experimental conditions or in the presence of ethane as an OH quencher, the product distribution observed required α = (7 ± 1)% + (1.1 ± 0.7) × 10−4 × (T-298) for 250K < T < 300K (2σ uncertainty), independent of pressure (at pressures > 266 hPa) and consistent with current IUPAC recommendations.
中文翻译:

关于甲乙酮的光解支化比
通过终产物分析和主化学机理(MCM)进行的盒模型模拟,重新评估了甲乙酮(MEK)的光解支化比(α)。使用中心在285 nm或315 nm处的发光二极管,在一氧化氮和氧气存在下照射MEK,以产生过氧乙酸和过氧丙酸一氧化氮,CH 3 C(O)O 2 NO 2(PAN)和C 2 H 5 C (O)O 2 NO 2(PPN),通过气相色谱法定量。盒模型模拟表明,PPN部分是作为副产物从化学反应生成的,该化学反应是由羟基(OH)与MEK反应引发的。在NO X在有限的实验条件下或在乙烷作为OH淬灭剂的情况下, 对于250K <T <300K,观察到的产物分布要求α=(7±1)%+(1.1±0.7)×10 -4 ×(T-298) (不确定性为2σ),与压力无关(在大于266 hPa的压力下),并且与当前IUPAC建议一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号