Organic Materials Pub Date : 2021-04-01 , DOI: 10.1055/s-0041-1725046 Yusuke Ishigaki 1 , Kota Asai 1 , Takuya Shimajiri 1 , Tomoyuki Akutagawa 2 , Takanori Fukushima 3 , Takanori Suzuki 1
|
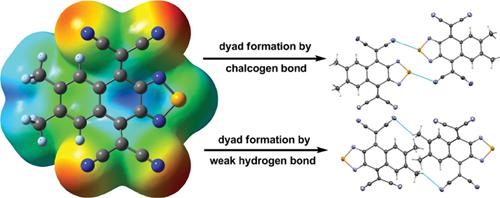
The crystal structures of a series of tetracyanonaphthoquinodimethanes fused with a selenadiazole or thiadiazole ring revealed that their molecular packing is determined mainly by two intermolecular interactions: chalcogen bond (ChB) and weak hydrogen bond (WHB). ChB between Se and a cyano group dictates the packing of selenadiazole derivatives, whereas the S-based ChB is much weaker and competes with WHB in thiadiazole analogues. This difference can be explained by different electrostatic potentials as revealed by density functional theory calculations. A proper molecular design that weakens WHB can change the contribution of ChB in determining the crystal packing of thiadiazole derivatives.
中文翻译:

硫属元素键与弱氢键:在确定[1,2,5]-硫族二唑-熔融四氰基萘甲喹啉甲烷晶体堆积中的变化贡献
与硒代二唑或噻二唑环稠合的一系列四氰基萘醌二甲烷的晶体结构表明,它们的分子堆积主要由两个分子间相互作用决定:硫属元素键(ChB)和弱氢键(WHB)。Se和氰基之间的ChB决定了硒代二唑衍生物的堆积,而基于S的ChB则弱得多,并且在噻二唑类似物中与WHB竞争。如密度泛函理论计算所揭示的,可以通过不同的静电势来解释这种差异。适当的减弱WHB的分子设计可以改变ChB在确定噻二唑衍生物的晶体堆积中的作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号