Solid State Sciences ( IF 3.5 ) Pub Date : 2021-03-29 , DOI: 10.1016/j.solidstatesciences.2021.106601 Dirk D. Zimmermann , Oliver Janka , Constantin Buyer , Rainer Niewa , Thomas Schleid
|
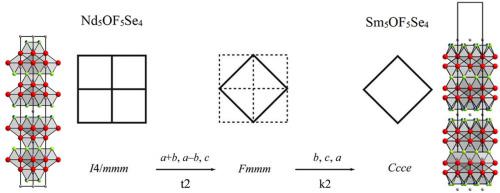
The formula Ln5OF5Se4 (Ln = Nd and Sm) represents a new composition for lanthanoid(III) oxide fluoride selenides. Nd5OF5Se4 could be seen as aristotype of this system, crystallizing in the tetragonal crystal system with space group I4/mmm and the lattice parameters a = 404.53(2) pm and c = 3400.02(18) pm (c/a = 8.4, Z = 2). In contrast to the aristotype, Sm5OF5Se4 exhibtis a symmetry reduction and adopts space group Ccce with lattice parameters of a = 564.85(3) pm, b = 3391.79(18) pm, c = 564.83(3) (b/c ≈ b/a = 6.0, pm Z = 4). Both compounds can be described by a layered arrangement of sheets, which are formed by the coordination polyhedra surrounding the three crystallographically independent Ln3+ cations in eight- and nine-fold coordination of anions. For reasons of electroneutrality one light-anion position has to be mixed-occupied with oxide (O2−) and fluoride (F−) anions for both compounds. Ln5OF5Se4, with edge-sharing [FLn4]11+ and [(O,F)Ln4]10.75+ tetrahedra linked to single and double layers separated by Se2− slabs, shows strong structural analogies to Er3OF3S2. In the case for Sm5OF5Se4 the absence of Sm2+ has been well supported by bond-valence calculations and XANES spectroscopy, while recorded DRS measurements were ambiguous owing to the red color of the substance.
中文翻译:

Nd 5 OF 5 Se 4和Sm 5 OF 5 Se 4:镧系元素的新的层状氧化物氟化硒
分子式Ln 5 OF 5 Se 4(Ln = Nd和Sm)代表一种镧系元素(III)氧化物氟化硒的新组成。Nd 5 OF 5 Se 4可以看作是该系统的贵族型,在空间群为I 4 / mmm且晶格参数为a = 404.53(2)pm和c = 3400.02(18)pm的四方晶体系统中结晶(c / a = 8.4,Z = 2)。与贵族型相反,Sm 5 OF 5 Se 4exhibtis对称还原,并采用空间群CCCE用的晶格参数一个= 564.85(3)时,b = 3391.79(18)时,Ç = 564.83(3)(b / ç ≈B / A = 6.0,PM ž = 4 )。两种化合物都可以通过薄片的分层排列来描述,这些薄片是由在阴离子的八倍和九倍配位下围绕三个晶体学独立的Ln 3+阳离子的配位多面体形成的。电中性的原因之一光阴离子位置必须混合占用与氧化物(O 2-)和氟化(F - )的阴离子两种化合物。LN 5作者5硒4,用了共享边缘的[F LN 4 ] 11+和[(O,F)LN 4 ] 10.75+四面体连接到单,双分离各层由硒2-板坯,示出了强结构类比来尔3的3 S 2。对于Sm 5 OF 5 Se 4,键合价计算和XANES光谱法很好地支持了Sm 2+的缺失,而记录的DRS测量结果由于该物质的红色而模棱两可。



























 京公网安备 11010802027423号
京公网安备 11010802027423号