Results in Chemistry ( IF 2.5 ) Pub Date : 2021-03-29 , DOI: 10.1016/j.rechem.2021.100128 Samantha A. Murphy , Oxana Kotova , Steve Comby , Thorfinnur Gunnlaugsson
|
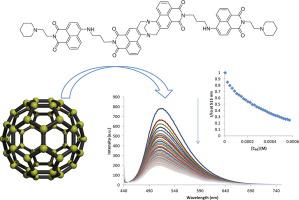
The synthesis and characterisation of two new 1,8-naphthalimide Tröger’s bases (TBNaps) 1 and 2 is described from the commercially available 4-chloro-1,8-naphthalic anhydride. These structures possess two additional 4-amino-1,8-naphthalimide (Naps) units, each of these ‘terminal’ Naps units have either 1-(2-aminoethyl)-pyrrolidine or 1-(2-aminoethyl)-piperidine moieties, that are conjugated to the TBNaps units via a short propyl spacer. The ground and the excited state properties of 1 and 2, as well as the bis-Nap precursor 10, were evaluated in a range of solvents that varied by polarity and hydrogen bonding ability. The UV–Vis absorption and the fluorescence emission spectra, demonstrated that all three compounds possess Internal Charge Transfer (ICT) excited state properties. Both 1 and 2 were designed as potential receptor molecules for the detection of C60 (buckminsterfullerene) where it was anticipated that C60 could possibly bind within the orthogonal cavity of the Tröger’s base’s, through additional interactions with the terminal Naps. While the titrations of 1 and 2 with C60 in a chlorobenzene:DMF (86:14) solvent mixture showed only minor changes in the absorption spectra, significant fluorescence quenching was observed for both 1 and 2. Analysis of the fluorescence data in terms of a Stern-Volmer plot, showed that the quenching involves a combination of both static and dynamic mechanisms.
中文翻译:

具有共轭4-氨基-1,8-萘二甲酰亚胺基团及其潜在富勒烯主体-客体配合物的荧光4-氨基-1,8-萘二甲酰亚胺Tröger碱基
从市售的4-氯-1,8-萘酐描述了两个新的1,8-萘二甲酰亚胺特罗格碱(TBNaps)1和2的合成和表征。这些结构具有两个额外的4-氨基-1,8-萘二甲酰亚胺(Naps)单元,每个这些“末端” Naps单元均具有1-(2-氨基乙基)-吡咯烷或1-(2-氨基乙基)-哌啶部分,通过一个短的丙基间隔基与TBNaps单元结合。1和2以及bis-Nap前体10的基态和激发态特性在一系列因极性和氢键结合能力而异的溶剂中进行了评估。UV-Vis吸收和荧光发射光谱表明,这三种化合物均具有内部电荷转移(ICT)激发态特性。既1和2被设计为C的检测电位的受体分子60(富勒烯),其中它被预期Ç 60可以在Tröger的碱的的正交空腔内可能结合,通过与终端另外的交互小睡。而用C 60滴定1和2在氯苯:DMF(86:14)溶剂混合物中,吸收光谱仅显示微小变化,对于1和2均观察到明显的荧光猝灭。用斯特恩-沃尔默图分析荧光数据表明,淬灭涉及静态和动态机制的结合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号