Current Organic Synthesis ( IF 1.7 ) Pub Date : 2021-05-01 , DOI: 10.2174/1570179417666201216161143 Jia-Qi Di 1 , Hao-Jie Wang 1 , Zhen-Shui Cui 1 , Jin-Yong Hu 1 , Zhan-Hui Zhang 1
|
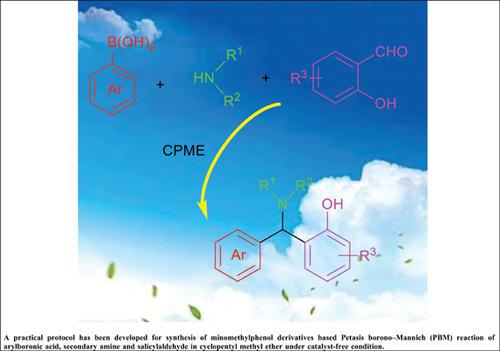
Objective: Aminomethylphenol molecules have wider applications in pharmaceuticals, agrochemicals, plant protection and promising functional materials. The development of an efficient and practical method to prepare this class of compound is highly desirable from both environmental and economical points of view.
Materials and Methods: In order to establish an effective synthetic method for preparing aminomethylphenol derivatives, the Petasis borono-Mannich reaction of salicylaldehyde, phenylboronic acid and 1,2,3,4- tetrahydroisoquinoline was selected as a model reaction. A variety of reaction conditions are investigated, including solvent and temperature. The generality and limitation of the established method were also evaluated.
Results and Discussion: It was found that model reaction can be carried out in cyclopentyl methyl ether at 80 oC under catalyst-free conditions. This protocol, with broad substrate applicability, the reaction of various arylboronic acid, secondary amine and salicylaldehyde proceeded smoothly under optimal reaction conditions to afford various aminomethylphenol derivatives in high yields. A practical, scalable, and high-yielding synthesis of aminomethylphenol derivatives was successfully accomplished.
Conclusion: A catalyst-free practical method for the synthesis of minomethylphenol derivatives based on Petasis borono–Mannich (PBM) reaction of various arylboronic acid, secondary amine and salicylaldehyde in cyclopentyl methyl ether has been developed. The salient features of this protocol are avoidance of any additive/catalyst and toxic organic solvents, use of cyclopentyl methyl ether as the reaction medium, clean reaction profiles, easy operation, and high to excellent yield.
中文翻译:

Petasis Borono-Mannich反应在环戊基甲基醚中无催化剂合成氨基甲基苯酚衍生物
目的:氨基甲基苯酚分子在医药、农化、植物保护和有前景的功能材料等方面有着广泛的应用。从环境和经济的角度来看,开发一种有效且实用的方法来制备此类化合物是非常需要的。
材料与方法:为了建立一种有效的合成氨甲基苯酚衍生物的合成方法,选择水杨醛、苯基硼酸和1,2,3,4-四氢异喹啉的Petasis硼-曼尼希反应作为模型反应。研究了多种反应条件,包括溶剂和温度。还评估了所建立方法的一般性和局限性。
结果与讨论:发现模型反应可以在环戊基甲基醚中在 80 oC 下在无催化剂条件下进行。该方案具有广泛的底物适用性,各种芳基硼酸、仲胺和水杨醛的反应在最佳反应条件下顺利进行,以高产率得到各种氨基甲基苯酚衍生物。成功完成了氨甲基苯酚衍生物的实用、可扩展和高产合成。
结论:基于各种芳基硼酸、仲胺和水杨醛在环戊基甲基醚中的 Petasis borono-Mannich (PBM) 反应,开发了一种无催化剂的实用合成氨基甲基苯酚衍生物的方法。该协议的显着特点是避免使用任何添加剂/催化剂和有毒有机溶剂,使用环戊基甲基醚作为反应介质,反应曲线干净,操作简单,产率高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号