Journal of Structural Biology ( IF 3 ) Pub Date : 2021-03-29 , DOI: 10.1016/j.jsb.2021.107731 Debbie S Retnoningrum 1 , Hiromi Yoshida 2 , Muthia D Razani 1 , Rahmat Muliadi 1 , Vincencius F Meidianto 3 , Anita Artarini 1 , Wangsa T Ismaya 3
|
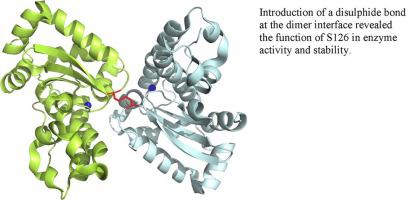
The dimeric form of manganese superoxide dismutase is instrumental for activity because each of the monomers provides amino acid residues participating in the enzymatic reaction. Hence, preventing dissociation of the dimer would maintain the enzymatic activity in detrimental conditions e.g. high temperature. To prevent dissociation of the dimer, a disulphide (S-S) bond was introduced at the dimer interface. In the wild type structure, S126 interacts with S126 of the other monomer. In the presented work, a mutant was designed with an S126C substitution. The crystal structure of the S126C mutant showed that only 50–70% of monomers formed the S-S bond. This observed imperfect S-S bonding was likely caused by photolytic S-S bond breakage mediated by the neighbouring tryptophan residue. In the wild type, S126 is located facing W163 and forms a water-mediated hydrogen bond with E164; W163 and E164 are crucial in the enzyme’s activity. The replacement of S126 by a cysteine residue lowered the activity of the enzyme by ~70%. S126 has never been considered to play a role in the enzyme’s activity or stability, thus the finding showed the importance of this residue.
中文翻译:

S126在金黄色葡萄球菌MnSOD活性和稳定性中的作用
锰超氧化物歧化酶的二聚体形式有助于活性,因为每个单体都提供参与酶促反应的氨基酸残基。因此,防止二聚体的解离将在有害条件下保持酶活性,例如高温。为了防止二聚体解离,在二聚体界面处引入了二硫 (SS) 键。在野生型结构中,S126 与其他单体的 S126 相互作用。在提出的工作中,设计了一个带有 S126C 替代的突变体。S126C 突变体的晶体结构表明只有 50-70% 的单体形成 SS 键。这种观察到的不完美的 SS 键可能是由相邻色氨酸残基介导的光解 SS 键断裂引起的。在野生型中,S126 面向 W163,与 E164 形成水介导的氢键;W163 和 E164 对酶的活性至关重要。用半胱氨酸残基取代 S126 使酶的活性降低了约 70%。S126 从未被认为在酶的活性或稳定性中起作用,



























 京公网安备 11010802027423号
京公网安备 11010802027423号