Journal of Structural Biology ( IF 3.0 ) Pub Date : 2021-03-27 , DOI: 10.1016/j.jsb.2021.107730 Yinshan Yang 1 , Jérome Gracy 1 , Nathalie Declerck 2 , Hélène Déméné 1
|
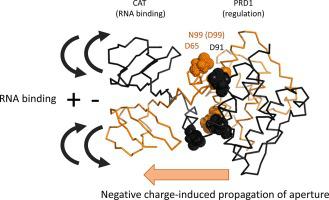
LicT is an antiterminator protein of the BglG family whose members are key players in the control of carbohydrate catabolism in bacteria. These antiterminators are generally composed of three modules, an N-terminal RNA-binding domain (CAT) followed by two homologous regulation modules (PRD1 and PRD2) that control the RNA binding activity of the effector domain via phosphorylation on conserved histidines. Although several structures of isolated domains of BglG-like proteins have been described, no structure containing CAT and at least one PRD simultaneously has yet been reported in an active state, precluding detailed understanding of signal transduction between modules. To fulfill this gap, we recently reported the complete NMR sequence assignment of a constitutively active mutant (D99N) CAT-PRD1*, which contains the effector domain and the first regulation domain of LicT. As a follow-up, we have determined and report here the 3D solution structure of this active, dimeric LicT construct (40 kDa). The structure reveals how the mutation constrains the PRD1 regulation domain into an active conformation which is transduced to CAT via a network of negatively charged residues belonging to PRD1 dimeric interface and to the linker region. In addition, our data support a model where BglG-type antitermination regulatory modules can only adopt a single conformation compatible with the active structure of the effector domain, regardless of whether activation is mediated by mutation on the first or second PRD. The linker between the effector and regulation modules appears to function as an adaptable hinge tuning the position of the functional modules.
中文翻译:

解析 D99N 抗终止子 LicT 蛋白的激活机制
LicT 是 BglG 家族的一种抗终止蛋白,其成员是控制细菌碳水化合物分解代谢的关键参与者。这些反终止子通常由三个模块组成,一个 N 端 RNA 结合域 (CAT),然后是两个同源调节模块 (PRD1 和 PRD2),它们通过对保守组氨酸的磷酸化来控制效应域的 RNA 结合活性。尽管已经描述了 BglG 样蛋白的分离结构域的几种结构,但尚未报道同时包含 CAT 和至少一个 PRD 的结构处于活动状态,从而排除了对模块之间信号转导的详细了解。为了填补这一空白,我们最近报道了组成型活性突变体 (D99N) CAT-PRD1* 的完整 NMR 序列分配,其中包含 LicT 的效应域和第一调节域。作为后续行动,我们在此确定并报告了这种活性二聚体 LicT 构建体 (40 kDa) 的 3D 解决方案结构。该结构揭示了突变如何将 PRD1 调节域限制为活性构象,该构象通过属于 PRD1 二聚体界面和接头区域的带负电残基网络转导至 CAT。此外,我们的数据支持一个模型,其中 BglG 型抗终止调节模块只能采用与效应域的活性结构兼容的单一构象,无论激活是由第一个或第二个 PRD 上的突变介导的。效应器和调节模块之间的链接器似乎起到了调节功能模块位置的适应性铰链的作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号