Journal of Structural Biology ( IF 3.0 ) Pub Date : 2021-03-26 , DOI: 10.1016/j.jsb.2021.107729 Teige R S Matthews-Palmer 1 , Nayim Gonzalez-Rodriguez 1 , Thomas Calcraft 2 , Signe Lagercrantz 1 , Tobias Zachs 1 , Xiu-Jun Yu 3 , Grzegorz J Grabe 3 , David W Holden 3 , Andrea Nans 4 , Peter B Rosenthal 4 , Sarah L Rouse 1 , Morgan Beeby 1
|
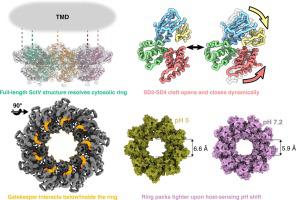
Bacterial type III secretion systems assemble the axial structures of both injectisomes and flagella. Injectisome type III secretion systems subsequently secrete effector proteins through their hollow needle into a host, requiring co-ordination. In the Salmonella enterica serovar Typhimurium SPI-2 injectisome, this switch is triggered by sensing the neutral pH of the host cytoplasm. Central to specificity switching is a nonameric SctV protein with an N-terminal transmembrane domain and a toroidal C-terminal cytoplasmic domain. A ‘gatekeeper’ complex interacts with the SctV cytoplasmic domain in a pH dependent manner, facilitating translocon secretion while repressing effector secretion through a poorly understood mechanism. To better understand the role of SctV in SPI-2 translocon-effector specificity switching, we purified full-length SctV and determined its toroidal cytoplasmic region’s structure using cryo-EM. Structural comparisons and molecular dynamics simulations revealed that the cytoplasmic torus is stabilized by its core subdomain 3, about which subdomains 2 and 4 hinge, varying the flexible outside cleft implicated in gatekeeper and substrate binding. In light of patterns of surface conservation, deprotonation, and structural motion, the location of previously identified critical residues suggest that gatekeeper binds a cleft buried between neighboring subdomain 4s. Simulations suggest that a local pH change from 5 to 7.2 stabilizes the subdomain 3 hinge and narrows the central aperture of the nonameric torus. Our results are consistent with a model of local pH sensing at SctV, where pH-dependent dynamics of SctV cytoplasmic domain affect binding of gatekeeper complex.
中文翻译:

沙门氏菌 SPI-2 注射体 SctV (SsaV) 细胞质结构域的结构及其对 pH 传感机制的影响
细菌 III 型分泌系统组装了注射体和鞭毛的轴向结构。注射体 III 型分泌系统随后通过其空心针将效应蛋白分泌到宿主中,需要协调。在肠沙门氏菌中血清型鼠伤寒 SPI-2 注射体,这个开关是通过感应宿主细胞质的中性 pH 值来触发的。特异性转换的核心是具有 N 端跨膜结构域和环形 C 端胞质结构域的非命名 SctV 蛋白。“看门人”复合物以 pH 依赖性方式与 SctV 细胞质结构域相互作用,促进易位子分泌,同时通过知之甚少的机制抑制效应分泌。为了更好地了解 SctV 在 SPI-2 易位子效应特异性转换中的作用,我们纯化了全长 SctV 并使用冷冻电镜确定了其环形细胞质区域的结构。结构比较和分子动力学模拟表明,细胞质环由其核心子结构域 3 稳定,子结构域 2 和 4 铰链,改变与看门人和基板结合有关的灵活外裂。根据表面保守、去质子化和结构运动的模式,先前确定的关键残基的位置表明,看门人结合了埋在相邻子域 4 之间的裂缝。模拟表明,从 5 到 7.2 的局部 pH 变化稳定了子域 3 铰链并缩小了非命名环的中心孔径。我们的结果与 SctV 的局部 pH 传感模型一致,其中 SctV 细胞质结构域的 pH 依赖性动态影响看门人复合物的结合。先前确定的关键残基的位置表明,看门人结合了埋在相邻子域 4 之间的裂缝。模拟表明,从 5 到 7.2 的局部 pH 变化稳定了子域 3 铰链并缩小了非命名环的中心孔径。我们的结果与 SctV 的局部 pH 传感模型一致,其中 SctV 细胞质结构域的 pH 依赖性动态影响看门人复合物的结合。先前确定的关键残基的位置表明,看门人结合了埋在相邻子域 4 之间的裂缝。模拟表明,从 5 到 7.2 的局部 pH 变化稳定了子域 3 铰链并缩小了非命名环的中心孔径。我们的结果与 SctV 的局部 pH 传感模型一致,其中 SctV 细胞质结构域的 pH 依赖性动态影响看门人复合物的结合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号