Journal of Rare Earths ( IF 5.2 ) Pub Date : 2021-03-21 , DOI: 10.1016/j.jre.2021.03.015 Meng Wang , Xiaowei Huang , Zongyu Feng , Chao Xia , Deliang Meng , Zonghe Yu
|
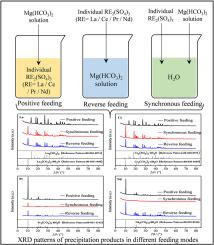
The precipitation of the water-leaching solution of Baotou mixed rare earth (RE) concentrate roasted with sulfuric acid using ammonium bicarbonate for producing RE carbonate produces a mass of ammonia-nitrogen wastewater because of the relatively low solubility of rare earth sulfate. To solve the serious problem of ammonia-nitrogen pollution, new precipitators need to be developed urgently so as to meet the requirements of environmental protection and impurities content of the product (SO42−<1.8 wt% in RE carbonates products). In this paper, we studied the effects of feeding modes on the behavior of SO42− during the preparation of light RE carbonate (RE = La, Ce, Pr, Nd) from their sulfate solutions using Mg(HCO3)2 as a precipitant. The results indicate that the contents of SO42− in the La and Ce precipitates using positive feeding mode exceed 16 wt% because of the formation of La2(CO3)2.15(SO4)0.85·4H2O and Ce2(CO3)2.15(SO4)0.85·3H2O, while those of the Pr and Nd precipitates are 4 wt%–5 wt% since they exist in the form of n-carbonate. The precipitates prepared using synchronous feeding mode are all RE carbonate with only 4 wt%–5 wt% of SO42− enclosed in the precipitation. The content of SO42− in the RE carbonate obtained using reverse feeding mode is the lowest. Among them, the content of SO42− in La precipitate is only 1.40 wt%. Both synchronous and reverse feeding modes can effectively reduce the content of SO42− in RE carbonate, which provides theoretical guidance for the preparation of qualified light RE carbonate products by Mg(HCO3)2 precipitation method.
中文翻译:

硫酸盐在Mg(HCO 3 ) 2沉淀法制备单一轻稀土碳酸盐中的行为
硫酸焙烧包头混合稀土(RE)精矿的水浸液沉淀使用碳酸氢铵生产碳酸稀土,由于硫酸稀土的溶解度较低,产生大量氨氮废水。为解决氨氮污染严重的问题,迫切需要开发新型沉淀器,以满足环保和产品杂质含量(稀土碳酸盐产品中SO 4 2− <1.8 wt%)的要求。在本文中,我们研究了在使用 Mg(HCO 3 ) 2从硫酸盐溶液制备轻质稀土碳酸盐(RE = La、Ce、Pr、Nd)过程中,进料模式对 SO 4 2-行为的影响作为沉淀剂。该结果表明,SO内容4 2-在La和Ce的沉淀物用正进给模式,因为La中形成的超过16%(重量)2(CO 3)2.15(SO 4)0.85 ·4H 2 O和Ce的2( CO 3 ) 2.15 (SO 4 ) 0.85 ·3H 2 O,而Pr和Nd沉淀物的含量为4wt%~5wt%,因为它们以n-碳酸盐的形式存在。使用同步进料模式制备的沉淀物都是稀土碳酸盐,只有 4 wt%–5 wt% SO 4 2−包裹在沉淀中。采用反向进料方式得到的稀土碳酸盐中SO 4 2-的含量最低。其中,La沉淀物中SO 4 2-的含量仅为1.40 wt%。同步和反向进料方式均能有效降低稀土碳酸盐中SO 4 2-的含量,为Mg(HCO 3 ) 2沉淀法制备合格的轻质稀土碳酸盐产品提供了理论指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号