当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stable Phase Diagram of the Quaternary Water–Salt System Li+, Na+, Rb+//SO42–·H2O at 298.2 and 323.2 K (P = 94.5 kPa)
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-03-24 , DOI: 10.1021/acs.jced.0c01019 Wannian Ying 1 , Ying Zeng 1, 2 , Xinxin Lv 1 , Hanlin Tong 1 , Wenzhang Zuo 1 , Pan Xu 1 , Xudong Yu 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-03-24 , DOI: 10.1021/acs.jced.0c01019 Wannian Ying 1 , Ying Zeng 1, 2 , Xinxin Lv 1 , Hanlin Tong 1 , Wenzhang Zuo 1 , Pan Xu 1 , Xudong Yu 1, 2
Affiliation
|
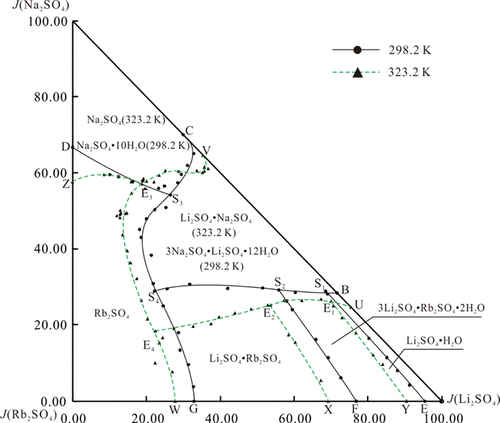
The stable phase equilibrium of the quaternary system Li+, Na+, Rb+// SO42– – H2O at T = 298.2 and 323.2 K (P = 94.5 kPa) was studied by an isothermal dissolution equilibrium method. The solubilities, density, and refractive index of equilibrated solution were determined. It is found that the projected phase diagrams of the quaternary system at 298.2 and 323.2 K (P = 94.5 kPa) both contain four invariant points, nine univariant curves, and six crystallization regions. By comparing the phase diagrams at 298.2 and 323.2 K, it is found that at 298.2 K, the region of single salt Rb2SO4 is the largest one and that of Li2SO4·H2O is the smallest one. At T = 323.2 K, the single salt Na2SO4·10H2O transforms into Na2SO4 and the lithium sodium double salt is transformed from 3Na2SO4·Li2SO4·12H2O to Li2SO4·Na2SO4. Meanwhile, the maximum phase region shifts from Rb2SO4 to Li2SO4·Na2SO4. This indicates that with the rise of temperature, the solubilities of Rb2SO4 and Li2SO4·Na2SO4 changed significantly and the lithium sodium double salt will be crystallized out from the system in the form of Li2SO4·Na2SO4, with a higher lithium sodium ratio. In other words, it is unfavorable to extract rubidium sulfate from sulfate brine, while it has obvious promoting effects on extracting lithium sulfate.
中文翻译:

四元水盐系统Li +,Na +,Rb + // SO 4 2– ·H 2 O在298.2和323.2 K时的稳定相图(P = 94.5 kPa)
采用等温溶解平衡法研究了四元体系Li +,Na +,Rb + // SO 4 2- – H 2 O在T = 298.2和323.2 K(P = 94.5 kPa)时的稳定相平衡。测定平衡溶液的溶解度,密度和折射率。发现在298.2和323.2 K(P = 94.5 kPa)处的四元体系投影相图都包含四个不变点,九个单变量曲线和六个结晶区域。通过比较在298.2 K和323.2 K处的相图,可以发现在298.2 K处,单盐Rb 2 SO的区域4是最大的,Li 2 SO 4 ·H 2 O是最小的。在T = 323.2 K时,单盐Na 2 SO 4 ·10H 2 O转化为Na 2 SO 4,锂钠复盐从3Na 2 SO 4 ·Li 2 SO 4 ·12H 2 O转化为Li 2 SO 4。 ·Na 2 SO 4。同时,最大相区从Rb 2 SO 4变为Li 2SO 4 ·Na 2 SO 4。这表明随着温度的升高,Rb 2 SO 4和Li 2 SO 4 ·Na 2 SO 4的溶解度发生明显变化,锂钠复盐将以Li 2 SO 4 ·的形式从体系中结晶出来。Na 2 SO 4,具有较高的锂钠比。换句话说,不利的是从硫酸盐盐水中提取硫酸rub,同时对提取硫酸锂具有明显的促进作用。
更新日期:2021-04-08
中文翻译:

四元水盐系统Li +,Na +,Rb + // SO 4 2– ·H 2 O在298.2和323.2 K时的稳定相图(P = 94.5 kPa)
采用等温溶解平衡法研究了四元体系Li +,Na +,Rb + // SO 4 2- – H 2 O在T = 298.2和323.2 K(P = 94.5 kPa)时的稳定相平衡。测定平衡溶液的溶解度,密度和折射率。发现在298.2和323.2 K(P = 94.5 kPa)处的四元体系投影相图都包含四个不变点,九个单变量曲线和六个结晶区域。通过比较在298.2 K和323.2 K处的相图,可以发现在298.2 K处,单盐Rb 2 SO的区域4是最大的,Li 2 SO 4 ·H 2 O是最小的。在T = 323.2 K时,单盐Na 2 SO 4 ·10H 2 O转化为Na 2 SO 4,锂钠复盐从3Na 2 SO 4 ·Li 2 SO 4 ·12H 2 O转化为Li 2 SO 4。 ·Na 2 SO 4。同时,最大相区从Rb 2 SO 4变为Li 2SO 4 ·Na 2 SO 4。这表明随着温度的升高,Rb 2 SO 4和Li 2 SO 4 ·Na 2 SO 4的溶解度发生明显变化,锂钠复盐将以Li 2 SO 4 ·的形式从体系中结晶出来。Na 2 SO 4,具有较高的锂钠比。换句话说,不利的是从硫酸盐盐水中提取硫酸rub,同时对提取硫酸锂具有明显的促进作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号