Journal of Energy Chemistry ( IF 14.0 ) Pub Date : 2021-03-26 , DOI: 10.1016/j.jechem.2021.03.029 Xing Yang Wu , Zhiyuan Tang , Xiaoxu Zhao , Xin Luo , Stephen John Pennycook , Song Ling Wang
|
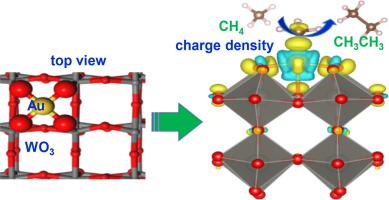
Gold (Au) as co-catalyst is remarkable for activating methane (CH4), especially atomically dispersed Au with maximized exposing active sites and specific electronic structure. Furthermore, singlet oxygen (1O2) typically manifests a mild redox capacity with a high selectivity to attack organic substrates. Peroxomonosulfate (PMS) favors to produce oxidative species 1O2 during the photocatalytic reactions. Thus, combining atomic Au as co-catalyst and 1O2 as oxidant is an effective strategy to selectively convert CH4. Herein, we synthesized atomically dispersed Au on WO3 (Au/WO3), where Au was in the forms of single atoms and clusters. At room temperature, such Au/WO3 exhibited enhanced photocatalytic conversion of CH4 to CH3CH3 with a selectivity, up to 94%, under visible light. The radicals-pathway mechanism of CH4 coupling has also been investigated through detection and trapping experiment of active species. Theoretical calculations further interpret the electronic structure of Au/WO3 and tip-enhanced local electric field at the Au sites for promoting CH4 conversion.
中文翻译:

在WO 3上原子分散的Au在甲烷和乙烷之间的可见光驱动的室温偶联
金(Au)作为助催化剂对于活化甲烷(CH 4)尤其有效,尤其是原子分散的Au,具有最大程度的暴露活性位点和特定的电子结构。此外,单线态氧(1 O 2)通常表现出中等的氧化还原能力,对有机底物具有高选择性。过氧单硫酸盐(PMS)有助于在光催化反应过程中产生氧化性物种1 O 2。因此,结合原子Au作为助催化剂和1 O 2作为氧化剂是选择性转化CH 4的有效策略。在这里,我们在WO 3(Au / WO 3),其中Au是单原子和簇的形式。在室温下,这种Au / WO 3在可见光下显示出CH 4向CH 3 CH 3的光催化转化增强,选择性高达94%。通过检测和捕获活性物种,还研究了CH 4偶联的自由基途径。理论计算进一步解释了Au / WO 3的电子结构以及Au位置处尖端增强的局部电场,以促进CH 4转化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号