当前位置:
X-MOL 学术
›
ACS ES&T Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption of Gaseous Mercury for Engineering Optimization: From Macrodynamics to Adsorption Kinetics and Thermodynamics
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2021-03-25 , DOI: 10.1021/acsestengg.1c00010 Qinyuan Hong 1 , Haomiao Xu 1 , Jiaxing Li 1 , Lu Tong 1 , Zhenxuan Tang 1 , Yuanxiang Xu 1 , Wenjun Huang 1 , Zan Qu 1, 2 , Naiqiang Yan 1, 2
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2021-03-25 , DOI: 10.1021/acsestengg.1c00010 Qinyuan Hong 1 , Haomiao Xu 1 , Jiaxing Li 1 , Lu Tong 1 , Zhenxuan Tang 1 , Yuanxiang Xu 1 , Wenjun Huang 1 , Zan Qu 1, 2 , Naiqiang Yan 1, 2
Affiliation
|
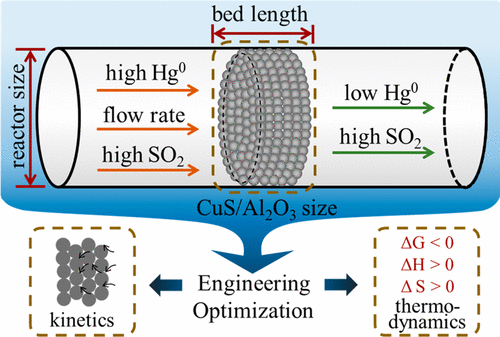
Adsorption is one of the most promising methods for gaseous mercury (Hg0) uptake from industrial flue gas, and the designing and synthesis of a sorbent are of significance for utilization. However, for a pilot-scale experiment, the macrodynamics, adsorption kinetics, and thermodynamics should be optimized before real applications. This study designed a scale-up fixed-bed reaction unit that worked with CuS-coated Al2O3 sorbent to investigate the influence of the gaseous diffusion, particle size, and wall effect on Hg0 removal performances. The results showed that the lower gas flow rate enhanced the mercury removal efficiencies. The combination of CuS/Al2O3 (1–2 mm) and a reaction tube (20 mm) mitigated the influence of size and wall effects, which maintained nearly complete mercury removal over 10 h under at 80 °C and had the average adsorption rate of 0.6 μg g–1 min–1. Moreover, CuS/Al2O3 possessed a higher SO2 resistance under a 1%–6% concentration range, guaranteeing a real application under SO2-rich industrial gas. The Hg0 adsorption was controlled by external diffusion processes based on the kinetic analysis. The negative ΔG (−30.71 to approximately −38.93 kJ mol–1), positive ΔS (123.39–138.80 J (mol K)−1), and positive ΔH (5.65–12.86 kJ mol–1) inferred the spontaneous, irreversible, and endothermic progress of Hg0 adsorption. The above key parameters have guiding significance for sorbent preparation and bed design in subsequent expanded applications.
中文翻译:

气态汞的吸附以进行工程优化:从宏观动力学到吸附动力学和热力学
吸附是从工业烟道气中吸收气态汞(Hg 0)的最有前途的方法之一,吸附剂的设计和合成对利用具有重要意义。但是,对于中试规模的实验,应在实际应用之前优化宏观动力学,吸附动力学和热力学。这项研究设计了一个放大的固定床反应装置,该装置与CuS包覆的Al 2 O 3吸附剂一起工作,以研究气体扩散,粒径和壁效应对Hg 0去除性能的影响。结果表明,较低的气体流速提高了除汞效率。CuS / Al 2 O 3的组合(1-2 mm)和反应管(20 mm)减轻了尺寸和壁效应的影响,在80°C的温度下,在10 h内汞保持几乎完全去除,平均吸附速率为0.6μgg –1分钟–1。此外,CuS / Al 2 O 3在1%–6%的浓度范围内具有较高的SO 2耐受性,从而保证了在富含SO 2的工业气体中的实际应用。基于动力学分析,通过外部扩散过程控制Hg 0的吸附。负ΔG(−30.71至约−38.93 kJ mol –1),正ΔS(123.39–138.80 J(mol K)-1)和正ΔH(5.65–12.86 kJ mol –1)推断Hg 0吸附的自发,不可逆和吸热过程。以上关键参数对于后续扩展应用中吸附剂的制备和床的设计具有指导意义。
更新日期:2021-05-14
中文翻译:

气态汞的吸附以进行工程优化:从宏观动力学到吸附动力学和热力学
吸附是从工业烟道气中吸收气态汞(Hg 0)的最有前途的方法之一,吸附剂的设计和合成对利用具有重要意义。但是,对于中试规模的实验,应在实际应用之前优化宏观动力学,吸附动力学和热力学。这项研究设计了一个放大的固定床反应装置,该装置与CuS包覆的Al 2 O 3吸附剂一起工作,以研究气体扩散,粒径和壁效应对Hg 0去除性能的影响。结果表明,较低的气体流速提高了除汞效率。CuS / Al 2 O 3的组合(1-2 mm)和反应管(20 mm)减轻了尺寸和壁效应的影响,在80°C的温度下,在10 h内汞保持几乎完全去除,平均吸附速率为0.6μgg –1分钟–1。此外,CuS / Al 2 O 3在1%–6%的浓度范围内具有较高的SO 2耐受性,从而保证了在富含SO 2的工业气体中的实际应用。基于动力学分析,通过外部扩散过程控制Hg 0的吸附。负ΔG(−30.71至约−38.93 kJ mol –1),正ΔS(123.39–138.80 J(mol K)-1)和正ΔH(5.65–12.86 kJ mol –1)推断Hg 0吸附的自发,不可逆和吸热过程。以上关键参数对于后续扩展应用中吸附剂的制备和床的设计具有指导意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号