当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface reaction kinetics of the methanol synthesis and the water gas shift reaction on Cu/ZnO/Al2O3
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2021-3-19 , DOI: 10.1039/d1re00040c Bruno Lacerda de Oliveira Campos 1, 2, 3, 4 , Karla Herrera Delgado 1, 2, 3, 4 , Stefan Wild 1, 2, 3, 4 , Felix Studt 1, 2, 3, 4, 5 , Stephan Pitter 1, 2, 3, 4 , Jörg Sauer 1, 2, 3, 4
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2021-3-19 , DOI: 10.1039/d1re00040c Bruno Lacerda de Oliveira Campos 1, 2, 3, 4 , Karla Herrera Delgado 1, 2, 3, 4 , Stefan Wild 1, 2, 3, 4 , Felix Studt 1, 2, 3, 4, 5 , Stephan Pitter 1, 2, 3, 4 , Jörg Sauer 1, 2, 3, 4
Affiliation
|
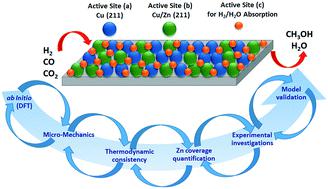
A three-site mean-field extended microkinetic model was developed based on ab initio DFT calculations from the literature, in order to simulate the conversion of syngas (H2/CO/CO2) to methanol on Cu (211) and Cu/Zn (211). The reaction network consists of 25 reversible reactions, including CO and CO2 hydrogenation to methanol and the water-gas shift reaction. Catalyst structural changes are also considered in the model. Experiments were performed in a plug flow reactor on Cu/ZnO/Al2O3 at various gas hourly space velocities (24–40 L h−1 gcat−1), temperatures (210–260 °C), pressures (40–60 bar), hydrogen feed concentrations (35–60% v/v), CO feed concentrations (3–30% v/v), and CO2 feed concentrations (0–20% v/v). These experiments, together with experimental data from the literature, were used for a broad validation of the model (a total of 690 points), which adequately reproduced the measurements. A degree of rate control analysis showed that the hydrogenation of formic acid is the major rate controlling step, and formate is the most sensitive surface species. The developed model contributes to the understanding of the reaction kinetics, and should be applicable for industrial processes (e.g. scale-up and optimization).
中文翻译:

Cu / ZnO / Al2O3上甲醇合成和水煤气变换反应的表面反应动力学
基于文献的从头算DFT计算,开发了一种三位平均场扩展微动力学模型,以模拟合成气(H 2 / CO / CO 2)在Cu(211)和Cu / Zn上转化为甲醇(211)。反应网络由25个可逆反应组成,包括CO和CO 2加氢成甲醇以及水煤气变换反应。该模型中还考虑了催化剂的结构变化。实验是在活塞流反应器中以各种气体时空速(24–40 L h -1 g cat -1的Cu / ZnO / Al 2 O 3)进行的),温度(210–260°C),压力(40–60 bar),氢气进料浓度(35–60%v / v),CO进料浓度(3–30%v / v)和CO 2进料浓度(0–20%v / v)。这些实验与文献中的实验数据一起用于模型的广泛验证(共690个点),从而充分再现了测量结果。一定程度的速率控制分析表明,甲酸加氢是主要的速率控制步骤,甲酸是最敏感的表面物质。所开发的模型有助于理解反应动力学,并且应适用于工业过程(例如放大和优化)。
更新日期:2021-03-25
中文翻译:

Cu / ZnO / Al2O3上甲醇合成和水煤气变换反应的表面反应动力学
基于文献的从头算DFT计算,开发了一种三位平均场扩展微动力学模型,以模拟合成气(H 2 / CO / CO 2)在Cu(211)和Cu / Zn上转化为甲醇(211)。反应网络由25个可逆反应组成,包括CO和CO 2加氢成甲醇以及水煤气变换反应。该模型中还考虑了催化剂的结构变化。实验是在活塞流反应器中以各种气体时空速(24–40 L h -1 g cat -1的Cu / ZnO / Al 2 O 3)进行的),温度(210–260°C),压力(40–60 bar),氢气进料浓度(35–60%v / v),CO进料浓度(3–30%v / v)和CO 2进料浓度(0–20%v / v)。这些实验与文献中的实验数据一起用于模型的广泛验证(共690个点),从而充分再现了测量结果。一定程度的速率控制分析表明,甲酸加氢是主要的速率控制步骤,甲酸是最敏感的表面物质。所开发的模型有助于理解反应动力学,并且应适用于工业过程(例如放大和优化)。









































 京公网安备 11010802027423号
京公网安备 11010802027423号