当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective hydrogenation of oleic acid to fatty alcohols over a Rh–Sn–B/Al2O3 catalyst: kinetics and optimal reaction conditions
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2021-3-23 , DOI: 10.1039/d0re00488j Cristhian A. Fonseca Benítez 1, 2, 3 , Vanina A. Mazzieri 1, 2, 3 , Carlos R. Vera 1, 2, 3 , Viviana M. Benitez 1, 2, 3 , Carlos L. Pieck 1, 2, 3
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2021-3-23 , DOI: 10.1039/d0re00488j Cristhian A. Fonseca Benítez 1, 2, 3 , Vanina A. Mazzieri 1, 2, 3 , Carlos R. Vera 1, 2, 3 , Viviana M. Benitez 1, 2, 3 , Carlos L. Pieck 1, 2, 3
Affiliation
|
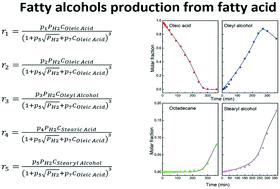
The selective hydrogenation of oleic acid to oleyl alcohol over a Rh(1 wt%)–Sn(4 wt%)–B/Al2O3 catalyst was studied. A comprehensive set of experimental data was used for elucidating the reaction mechanism. In the range of reaction conditions of this work, the optimal conditions found were 290 °C and 2 MPa, with a yield of 82–83% of oleyl alcohol. Kinetic models were written which considered the whole network of reactions taking place: double bond hydrogenation, acid hydrogenation to alcohol, and esterification of acids and alcohols. Different combinations of elementary steps led to the formulation of a big number of models. Models posing the surface reactions as rate-limiting fitted the data better. Adsorption of the acids, the alcohols or hydrogen was not rate-limiting. The best-fit model had the following hypotheses: (i) only one kind of adsorption site is needed for all species and reactions; (ii) H2 is dissociatively adsorbed; (iii) fatty molecules are adsorbed on only one site; (iv) pairwise insertion of H to fatty molecules is the rate-limiting step; (v) reduction of the carboxylate group occurs via an aldehyde intermediate that is subsequently hydrogenated to the corresponding alcohol; (vii) hydrogen and oleic acid are the main adsorbates; (vii) heavy esters are formed but do not contribute as intermediates of the main mechanism.
中文翻译:

在Rh–Sn–B / Al2O3催化剂上将油酸选择性氢化为脂肪醇:动力学和最佳反应条件
在Rh(1 wt%)– Sn(4 wt%)– B / Al 2 O 3上将油酸选择性氢化为油醇研究了催化剂。使用一组全面的实验数据来阐明反应机理。在这项工作的反应条件范围内,发现的最佳条件是290°C和2 MPa,油醇的收率为82–83%。编写了动力学模型,其中考虑了发生的整个反应网络:双键加氢,酸加氢成醇,以及酸和醇的酯化。基本步骤的不同组合导致大量模型的提出。将表面反应视为速率限制的模型可以更好地拟合数据。酸,醇或氢的吸附不受速率的限制。最佳拟合模型具有以下假设:(i)所有物种和反应仅需要一种吸附位点;(ii)H 2解离地吸附;(iii)脂肪分子仅吸附在一个部位上;(iv)将H成对插入脂肪分子是限速步骤;(v)羧酸酯基的还原是通过醛中间体发生的,该醛中间体随后被氢化为相应的醇;(vii)氢和油酸是主要吸附物;(vii)形成重酯,但不作为主要机理的中间体。
更新日期:2021-03-23
中文翻译:

在Rh–Sn–B / Al2O3催化剂上将油酸选择性氢化为脂肪醇:动力学和最佳反应条件
在Rh(1 wt%)– Sn(4 wt%)– B / Al 2 O 3上将油酸选择性氢化为油醇研究了催化剂。使用一组全面的实验数据来阐明反应机理。在这项工作的反应条件范围内,发现的最佳条件是290°C和2 MPa,油醇的收率为82–83%。编写了动力学模型,其中考虑了发生的整个反应网络:双键加氢,酸加氢成醇,以及酸和醇的酯化。基本步骤的不同组合导致大量模型的提出。将表面反应视为速率限制的模型可以更好地拟合数据。酸,醇或氢的吸附不受速率的限制。最佳拟合模型具有以下假设:(i)所有物种和反应仅需要一种吸附位点;(ii)H 2解离地吸附;(iii)脂肪分子仅吸附在一个部位上;(iv)将H成对插入脂肪分子是限速步骤;(v)羧酸酯基的还原是通过醛中间体发生的,该醛中间体随后被氢化为相应的醇;(vii)氢和油酸是主要吸附物;(vii)形成重酯,但不作为主要机理的中间体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号