Journal of Magnetic Resonance Open ( IF 1.5 ) Pub Date : 2021-03-18 , DOI: 10.1016/j.jmro.2021.100013 Ian R Stecker 1, 2 , Matthew S Freeman 2 , Sneha Sitaraman 3 , Chase S Hall 4 , Peter J Niedbalski 2, 4 , Alexandra J Hendricks 1, 2 , Emily P Martin 3 , Timothy E Weaver 3, 5 , Zackary I Cleveland 1, 2, 5
|
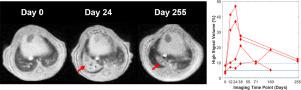
Structural remodeling in lung disease is progressive and heterogeneous, making temporally and spatially explicit information necessary to understand disease initiation and progression. While mouse models are essential to elucidate mechanistic pathways underlying disease, the experimental tools commonly available to quantify lung disease burden are typically invasive (e.g., histology). This necessitates large cross-sectional studies with terminal endpoints, which increases experimental complexity and expense. Alternatively, magnetic resonance imaging (MRI) provides information noninvasively, thus permitting robust, repeated-measures statistics. Although lung MRI is challenging due to low tissue density and rapid apparent transverse relaxation (T2* <1 ms), various imaging methods have been proposed to quantify disease burden. However, there are no widely accepted strategies for preclinical lung MRI. As such, it can be difficult for researchers who lack lung imaging expertise to design experimental protocols—particularly for novel mouse models. Here, we build upon prior work from several research groups to describe a widely applicable acquisition and analysis pipeline that can be implemented without prior preclinical pulmonary MRI experience. Our approach utilizes 3D radial ultrashort echo time (UTE) MRI with retrospective gating and lung segmentation is facilitated with a deep-learning algorithm. This pipeline was deployed to assess disease dynamics over 255 days in novel, transgenic mouse models of lung fibrosis based on disease-associated, loss-of-function mutations in Surfactant Protein-C. Previously identified imaging biomarkers (tidal volume, signal coefficient of variation, etc.) were calculated semi-automatically from these data, with an objectively-defined high signal volume identified as the most robust metric. Beyond quantifying disease dynamics, we discuss common pitfalls encountered in preclinical lung MRI and present systematic approaches to identify and mitigate these challenges. While the experimental results and specific pedagogical examples are confined to lung fibrosis, the tools and approaches presented should be broadly useful to quantify structural lung disease in a wide range of mouse models.
中文翻译:

在特征不佳的小鼠模型中量化肺部疾病严重程度和轨迹的临床前 MRI:使用来自新型肺纤维化转基因模型数据的教学示例
肺部疾病的结构重塑是渐进的和异质的,因此需要时间和空间明确的信息来了解疾病的发生和进展。虽然小鼠模型对于阐明疾病的潜在机制至关重要,但通常可用于量化肺部疾病负担的实验工具通常是侵入性的(例如,组织学)。这需要具有终端终点的大型横断面研究,这增加了实验的复杂性和费用。或者,磁共振成像 (MRI) 以无创方式提供信息,从而允许进行稳健、重复测量的统计数据。尽管肺 MRI 由于低组织密度和快速表观横向松弛(T 2* <1 ms),已经提出了各种成像方法来量化疾病负担。然而,临床前肺 MRI 还没有被广泛接受的策略。因此,对于缺乏肺部成像专业知识的研究人员来说,设计实验方案可能很困难,尤其是对于新型小鼠模型。在这里,我们以几个研究小组的先前工作为基础,描述了一个广泛适用的采集和分析管道,无需先前的临床前肺 MRI 经验即可实施。我们的方法利用具有回顾性门控的 3D 径向超短回波时间 (UTE) MRI,并且通过深度学习算法促进了肺分割。该管道用于评估基于疾病相关的新型肺纤维化转基因小鼠模型超过 255 天的疾病动态,表面活性剂蛋白-C 中的功能丧失突变。先前确定的成像生物标志物(潮气量、信号变异系数等)是根据这些数据半自动计算的,客观定义的高信号量被确定为最可靠的指标。除了量化疾病动态之外,我们还讨论了临床前肺部 MRI 中遇到的常见陷阱,并提出了识别和缓解这些挑战的系统方法。虽然实验结果和具体的教学实例仅限于肺纤维化,但所提供的工具和方法应该广泛用于量化各种小鼠模型中的结构性肺病。客观定义的高信号量被认为是最稳健的指标。除了量化疾病动态之外,我们还讨论了临床前肺部 MRI 中遇到的常见陷阱,并提出了识别和缓解这些挑战的系统方法。虽然实验结果和具体的教学实例仅限于肺纤维化,但所提供的工具和方法应该广泛用于量化各种小鼠模型中的结构性肺病。客观定义的高信号量被认为是最稳健的指标。除了量化疾病动态之外,我们还讨论了临床前肺部 MRI 中遇到的常见陷阱,并提出了识别和缓解这些挑战的系统方法。虽然实验结果和具体的教学实例仅限于肺纤维化,但所提供的工具和方法应该广泛用于量化各种小鼠模型中的结构性肺病。











































 京公网安备 11010802027423号
京公网安备 11010802027423号