Forensic Chemistry ( IF 2.6 ) Pub Date : 2021-03-18 , DOI: 10.1016/j.forc.2021.100334 Koichi Saito , Rieko Saito , Rie Ito
|
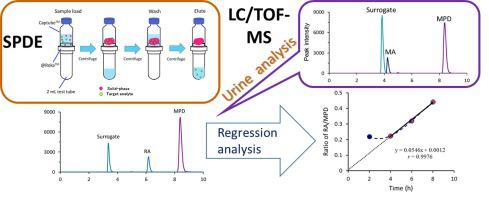
A simple and rapid analytical method for the determination of methamphetamine (MA) and methylphenidate (MPD) in urine was developed, which involves liquid chromatography/time-of-flight mass spectrometry (LC/TOF-MS) and solid-phase dispersive extraction (SPDE). The developed method could be used in pharmacokinetic studies. Oasis® WCX gel was used in SPDE. LC separation was carried out on a Poroshell 120 EC-C18 column (100 mm × 4.6 mm I.D., 2.7 µm) using a mixture (30:70) of methanol/10 mM ammonium formate aqueous solution (pH 3.0). The calibration curves showed good linearity in the range of 2 to 100 ng/mL for MA and 0.5 to 200 ng/mL for MPD. The limits of detection were 0.5 ng/mL and 0.1 ng/mL, and the limits of quantification were 2 ng/mL and 0.5 ng/mL for MA and MPD, respectively. The average recoveries of MA and MPD from urine samples spiked with MA at 5 and 50 ng/mL and MPD at 1 and 100 ng/mL were 93.9–94.8% (RSD: 1.6–2.1%) and 91.5–106.5% (RSD: 1.3–3.0%), respectively. Periodic analyses of urine samples collected from a volunteer receiving clinical doses of MPD revealed the presence of ritalinic acid (RA), an inactive major metabolite of MPD. Furthermore, when the RA/MPD ratios corrected by the urinary creatinine level were plotted against time, the regression curve showed linearity after 4 h. Pharmacokinetic study revealed that the extrapolated line of the regression line reached the origin in the graph, suggesting that the regression line could be used to calculate the dosage and timing of administration.
中文翻译:

液相色谱/飞行时间质谱联用固相分散萃取法测定尿液中的甲基苯丙胺和哌醋甲酯及其药代动力学应用
建立了一种简便快速的测定尿液中甲基苯丙胺(MA)和哌醋甲酯(MPD)的分析方法,该方法涉及液相色谱/飞行时间质谱(LC / TOF-MS)和固相分散萃取( SPDE)。所开发的方法可用于药代动力学研究。WCX凝胶用于SPDE。使用甲醇/ 10 mM甲酸铵水溶液(pH 3.0)的混合物(30:70)在Poroshell 120 EC-C18色谱柱(100 mm×4.6 mm内径,2.7 µm)上进行LC分离。校准曲线显示出良好的线性,MA的线性范围为2到100 ng / mL,MPD的线性范围为0.5到200 ng / mL。MA和MPD的检出限分别为0.5 ng / mL和0.1 ng / mL,定量限分别为2 ng / mL和0.5 ng / mL。分别以5和50 ng / mL的MA以及1和100 ng / mL的MPD加标的尿液样品中MA和MPD的平均回收率分别为93.9–94.8%(RSD:1.6–2.1%)和91.5–106.5%(RSD: 1.3–3.0%)。对接受临床剂量MPD的志愿者收集的尿液样本进行的定期分析显示,存在利他林酸(RA)(一种非活性的MPD主要代谢物)。此外,当用尿肌酐水平校正的RA / MPD比值相对于时间作图时,回归曲线显示4小时后呈线性。药代动力学研究表明,回归线的外推线已到达图中的原点,这表明该回归线可用于计算给药剂量和给药时间。分别。对接受临床剂量MPD的志愿者收集的尿液样本进行的定期分析显示,存在利他林酸(RA)(一种非活性的MPD主要代谢物)。此外,当用尿肌酐水平校正的RA / MPD比值相对于时间作图时,回归曲线显示4小时后呈线性。药代动力学研究表明,回归线的外推线已到达图中的原点,这表明该回归线可用于计算给药剂量和给药时间。分别。对接受临床剂量MPD的志愿者收集的尿液样本进行的定期分析显示,存在利他林酸(RA)(一种非活性的MPD主要代谢物)。此外,当用尿肌酐水平校正的RA / MPD比值相对于时间作图时,回归曲线显示4小时后呈线性。药代动力学研究表明,回归线的外推线已到达图中的原点,这表明该回归线可用于计算给药剂量和给药时间。4 h后回归曲线呈线性。药代动力学研究表明,回归线的外推线已到达图中的原点,这表明该回归线可用于计算给药剂量和给药时间。4 h后回归曲线呈线性。药代动力学研究表明,回归线的外推线已到达图中的原点,这表明该回归线可用于计算给药剂量和给药时间。











































 京公网安备 11010802027423号
京公网安备 11010802027423号