当前位置:
X-MOL 学术
›
J. Inherit. Metab. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In a mouse model of INCL reduced S-palmitoylation of cytosolic thioesterase APT1 contributes to microglia proliferation and neuroinflammation
Journal of Inherited Metabolic Disease ( IF 4.2 ) Pub Date : 2021-03-19 , DOI: 10.1002/jimd.12379 Tamal Sadhukhan 1 , Maria B Bagh 1 , Abhilash P Appu 1 , Avisek Mondal 1 , Wei Zhang 2 , Aiyi Liu 2 , Anil B Mukherjee 1
Journal of Inherited Metabolic Disease ( IF 4.2 ) Pub Date : 2021-03-19 , DOI: 10.1002/jimd.12379 Tamal Sadhukhan 1 , Maria B Bagh 1 , Abhilash P Appu 1 , Avisek Mondal 1 , Wei Zhang 2 , Aiyi Liu 2 , Anil B Mukherjee 1
Affiliation
|
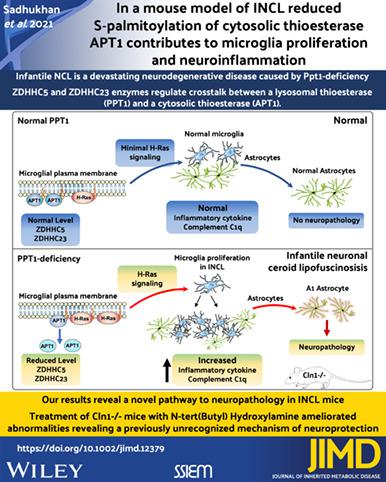
S-palmitoylation is a reversible posttranslational modification in which a 16-carbon saturated fatty acid (generally palmitate) is attached to specific cysteine residues in polypeptides via thioester linkage. Dynamic S-palmitoylation (palmitoylation-depalmitoylation), like phosphorylation-dephosphorylation, regulates the function of numerous proteins, especially in the brain. While a family of 23 palmitoyl-acyl transferases (PATS), commonly known as ZDHHCs, catalyze S-palmitoylation of proteins, the thioesterases, localized either in the cytoplasm (eg, APT1) or in the lysosome (eg, PPT1) mediate depalmitoylation. Previously, we reported that APT1 requires dynamic S-palmitoylation for shuttling between the cytosol and the plasma membrane. APT1 depalmitoylated H-Ras to regulate its signaling pathway that stimulates cell proliferation. Although we demonstrated that APT1 catalyzed its own depalmitoylation, the ZDHHC(s) that S-palmitoylated APT1 had remained unidentified. We report here that ZDHHC5 and ZDHHC23 catalyze APT1 S-palmitoylation. Intriguingly, lysosomal Ppt1-deficiency in Cln1−/− mouse, a reliable animal model of INCL, markedly reduced ZDHHC5 and ZDHHC23 levels. Remarkably, in the brain of these mice decreased ZDHHC5 and ZDHHC23 levels suppressed membrane-bound APT1, thereby, increasing plasma membrane-localized H-Ras, which activated its signaling pathway stimulating microglia proliferation. Increased inflammatory cytokines produced by microglia together with increased complement C1q level contributed to the transformation of astrocytes to neurotoxic A1 phenotype. Importantly, neuroinflammation was ameliorated by treatment of Cln1−/− mice with a PPT1-mimetic small molecule, N-tert(Butyl)hydroxylamine (NtBuHA). Our results revealed a novel pathway to neuropathology in an INCL mouse model and uncovered a previously unrecognized mechanism of the neuroprotective actions of NtBuHA and its potential as a drug target.
中文翻译:

在 INCL 小鼠模型中,胞质硫酯酶 APT1 的 S-棕榈酰化降低有助于小胶质细胞增殖和神经炎症
S-棕榈酰化是一种可逆的翻译后修饰,其中 16 个碳的饱和脂肪酸(通常是棕榈酸酯)通过硫酯键连接到多肽中的特定半胱氨酸残基上。动态 S-棕榈酰化 (palmitoylation-depalmitoylation) 与磷酸化-去磷酸化一样,调节多种蛋白质的功能,尤其是在大脑中。虽然通常称为 ZDHHCs 的 23 种棕榈酰-酰基转移酶 (PATS) 家族催化蛋白质的 S-棕榈酰化,但位于细胞质 (例如 APT1) 或溶酶体 (例如 PPT1) 中的硫酯酶介导去棕榈酰化。以前,我们报道过 APT1 需要动态 S-棕榈酰化才能在胞质溶胶和质膜之间穿梭。APT1 去棕榈酰化 H-Ras 以调节其刺激细胞增殖的信号通路。尽管我们证明了 APT1 催化了其自身的去棕榈酰化,但 S-棕榈酰化 APT1 的 ZDHHC(s) 仍未被识别。我们在此报告 ZDHHC5 和 ZDHHC23 催化 APT1 S-棕榈酰化。有趣的是,溶酶体 Ppt1 缺乏Cln1 -/-小鼠,一种可靠的 INCL 动物模型,显着降低了 ZDHHC5 和 ZDHHC23 水平。值得注意的是,在这些小鼠的大脑中,ZDHHC5 和 ZDHHC23 水平的降低抑制了膜结合的 APT1,从而增加了质膜定位的 H-Ras,从而激活了其信号通路刺激小胶质细胞增殖。小胶质细胞产生的炎性细胞因子增加以及补体 C1q 水平增加有助于星形胶质细胞转化为神经毒性 A1 表型。重要的是,通过治疗Cln1 -/-改善了神经炎症具有 PPT1 模拟小分子 N-叔(丁基)羟胺 (NtBuHA) 的小鼠。我们的研究结果揭示了 INCL 小鼠模型中神经病理学的新途径,并揭示了 NtBuHA 的神经保护作用的先前未被认识的机制及其作为药物靶点的潜力。
更新日期:2021-03-19
中文翻译:

在 INCL 小鼠模型中,胞质硫酯酶 APT1 的 S-棕榈酰化降低有助于小胶质细胞增殖和神经炎症
S-棕榈酰化是一种可逆的翻译后修饰,其中 16 个碳的饱和脂肪酸(通常是棕榈酸酯)通过硫酯键连接到多肽中的特定半胱氨酸残基上。动态 S-棕榈酰化 (palmitoylation-depalmitoylation) 与磷酸化-去磷酸化一样,调节多种蛋白质的功能,尤其是在大脑中。虽然通常称为 ZDHHCs 的 23 种棕榈酰-酰基转移酶 (PATS) 家族催化蛋白质的 S-棕榈酰化,但位于细胞质 (例如 APT1) 或溶酶体 (例如 PPT1) 中的硫酯酶介导去棕榈酰化。以前,我们报道过 APT1 需要动态 S-棕榈酰化才能在胞质溶胶和质膜之间穿梭。APT1 去棕榈酰化 H-Ras 以调节其刺激细胞增殖的信号通路。尽管我们证明了 APT1 催化了其自身的去棕榈酰化,但 S-棕榈酰化 APT1 的 ZDHHC(s) 仍未被识别。我们在此报告 ZDHHC5 和 ZDHHC23 催化 APT1 S-棕榈酰化。有趣的是,溶酶体 Ppt1 缺乏Cln1 -/-小鼠,一种可靠的 INCL 动物模型,显着降低了 ZDHHC5 和 ZDHHC23 水平。值得注意的是,在这些小鼠的大脑中,ZDHHC5 和 ZDHHC23 水平的降低抑制了膜结合的 APT1,从而增加了质膜定位的 H-Ras,从而激活了其信号通路刺激小胶质细胞增殖。小胶质细胞产生的炎性细胞因子增加以及补体 C1q 水平增加有助于星形胶质细胞转化为神经毒性 A1 表型。重要的是,通过治疗Cln1 -/-改善了神经炎症具有 PPT1 模拟小分子 N-叔(丁基)羟胺 (NtBuHA) 的小鼠。我们的研究结果揭示了 INCL 小鼠模型中神经病理学的新途径,并揭示了 NtBuHA 的神经保护作用的先前未被认识的机制及其作为药物靶点的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号