Journal of Structural Biology ( IF 3.0 ) Pub Date : 2021-03-18 , DOI: 10.1016/j.jsb.2021.107725 Dario Heymann 1 , Harini Mohanram 2 , Akshita Kumar 3 , Chandra S Verma 4 , Julien Lescar 5 , Ali Miserez 6
|
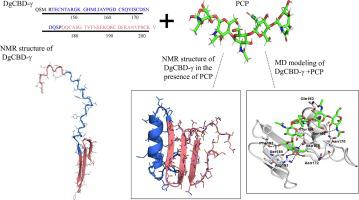
Chitin-binding proteins (CBPs) are a versatile group of proteins found in almost every organism on earth. CBPs are involved in enzymatic carbohydrate degradation and also serve as templating scaffolds in the exoskeleton of crustaceans and insects. One specific chitin-binding motif found across a wide range of arthropods’ exoskeletons is the “extended Rebers and Riddiford” consensus (R&R), whose mechanism of chitin binding remains unclear. Here, we report the 3D structure and molecular level interactions of a chitin-binding domain (CBD-γ) located in a CBP from the beak of the jumbo squid Dosidicus gigas. This CBP is one of four chitin-binding proteins identified in the beak mouthpart of D. gigas and is believed to interact with chitin to form a scaffold network that is infiltrated with a second set of structural proteins during beak maturation. We used solution state NMR spectroscopy to elucidate the molecular interactions between CBD-γ and the soluble chitin derivative pentaacetyl-chitopentaose (PCP), and find that folding of CBD-γ is triggered upon its interaction with PCP. To our knowledge, this is the first experimental 3D structure of a CBP containing the R&R consensus motif, which can be used as a template to understand in more details the role of the R&R motif found in a wide range of CBP-chitin complexes. The present structure also provides molecular information for biomimetic synthesis of graded biomaterials using aqueous-based chemistry and biopolymers.
中文翻译:

溶液核磁共振揭示的一致几丁质结合域的结构
几丁质结合蛋白 (CBP) 是在地球上几乎所有生物中发现的一组多功能蛋白质。CBPs 参与酶促碳水化合物降解,也可作为甲壳类动物和昆虫外骨骼的模板支架。在多种节肢动物的外骨骼中发现的一种特定的几丁质结合基序是“扩展的 Rebers 和 Riddiford”共识 (R&R),其几丁质结合的机制仍不清楚。在这里,我们报告了位于巨型鱿鱼Dosidicus gigas喙中的 CBP 中的几丁质结合域 (CBD-γ) 的 3D 结构和分子水平相互作用。这种 CBP 是在D. gigas的喙口器中鉴定的四种几丁质结合蛋白之一并且被认为与几丁质相互作用以形成支架网络,该支架网络在喙成熟期间被第二组结构蛋白浸润。我们使用溶液态核磁共振光谱来阐明 CBD-γ 和可溶性几丁质衍生物五乙酰壳五糖 (PCP) 之间的分子相互作用,并发现 CBD-γ 的折叠是在其与 PCP 相互作用时触发的。据我们所知,这是第一个包含 R&R 共有基序的 CBP 实验性 3D 结构,它可以用作模板来更详细地了解在广泛的 CBP-几丁质复合物中发现的 R&R 基序的作用。本结构还为使用水基化学和生物聚合物仿生合成分级生物材料提供了分子信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号